RNNR Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
|
Following the unexpected shutdown of the National Research Universal (NRU) reactor at Atomic Energy of Canada Limited's (AECL) Chalk River laboratories in Eastern Ontario on May 14, 2009, the House of Commons Standing Committee on Natural Resources (hereafter the Committee) held several meetings with the purpose of examining the consequences of the shutdown in Canada and abroad. After approximately 15 months of repair activities, the reactor was restarted on August 17, 2010. This report summarizes the Committee's study and presents recommendations to the Government of Canada regarding medical isotope production, supply and research in Canada, including alternatives to medical isotopes. The NRU reactor, which first came on stream in November 1957, serves three purposes:
The NRU has the capacity to be the largest global supplier of medical isotopes, and is one of the world's few reactors available for research and commercial use. It receives over 200 professors, students and industrial researchers annually, and, according to Dr. Dominic Ryan of the Canadian Institute for Neutron Scattering, has contributed to "50 years of Canadian leadership in nuclear science and technology".[3] Following the NRU shutdown, the Expert Review Panel on Medical Isotope Production (hereafter the Expert Panel) was established by the Ministers of Natural Resources and Health to examine ideas and proposals regarding the production of medical isotopes. The Expert Panel presented its final report to the government on November 30, 2009. This is not the first time that the NRU reactor has been shut down unexpectedly. In December 2007, the Canadian Nuclear Safety Commission (CNSC) shut down the reactor due to non conformity of safety measures, which triggered concerns about a possible global shortage in medical isotopes. The Parliament of Canada intervened by adopting a special bill that forced the return of the NRU reactor to service. Table 1 presents the operating and capital costs of the NRU reactor in 2000/01, 2004/05 and 2008/09, as provided by AECL. Table 1-NRU Operating and Capital Expenditures
Source: Document presented to the Committee on October 20, 2009 by Serge Dupont, Special Advisor to the Minister of Natural Resources on Nuclear Energy Policy. Prior to the NRU reactor outage, the federal government has been considering the restructuring of AECL, and there have been various inquiries regarding the overall state of the nuclear industry. While this report focuses on the NRU reactor shutdown and the consequential isotope shortage, these wider considerations have implications on medical research and the production of medical isotopes in Canada. Based on a wide-range of Canadian and international expertise, the report concludes the Committee's hearings on the subject to-date. On May 14, 2009, the NRU reactor shut down automatically due to a power outage. Subsequently, a heavy-water leak at 5 kilograms per hour was discovered, leading AECL to extend the shutdown until the problem was resolved. The leak was a result of corrosion at the base of the reactor's vessel. AECL testified that the heavy water was captured and stored in drums, and posed no health or safety risk to the public or the environment.[4] In addition, some evaporation from the leak resulted in tritium air emissions at levels "well below CNSC regulatory limits".[5] Following a repair process that lasted approximately 15 months, AECL reported on August 17, 2010 that the testing on the NRU reactor was complete, and that the reactor had started operating "at high power and could begin to create medical isotopes". On August 18, 2010, the first Molybdenum-99 (Mo-99) isotope following the restart was harvested from the NRU reactor. Finally, on August 25, 2010, AECL reported that the reactor had resumed "full production of medical isotopes".[6] In order to return the NRU reactor to service, AECL was faced with an unprecedented technical challenge since all inspection and repair activities in the reactor vessel had to be carried out by remote tooling through a 12-centimeter aperture, nine meters away from the corroded surface.[7] A three-phase program was established to carry out the NRU return-to-service plan:
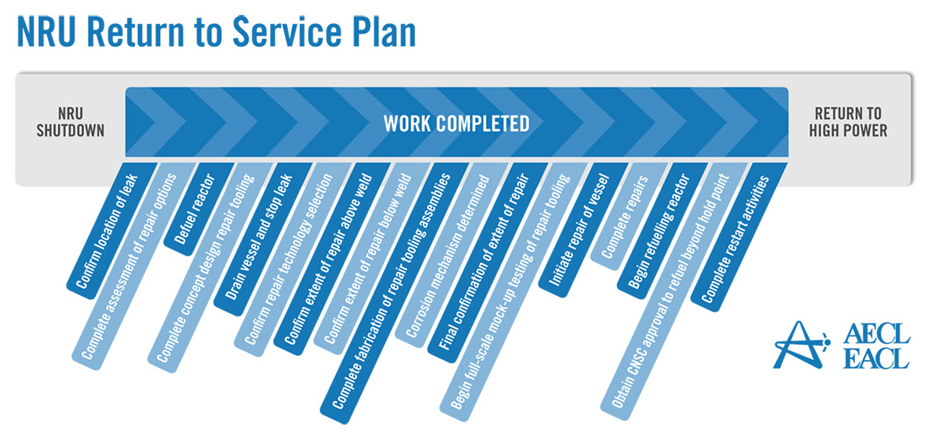
Figure 1 presents the NRU return-to-service plan. Figure 1: NRU Return to Service Plan
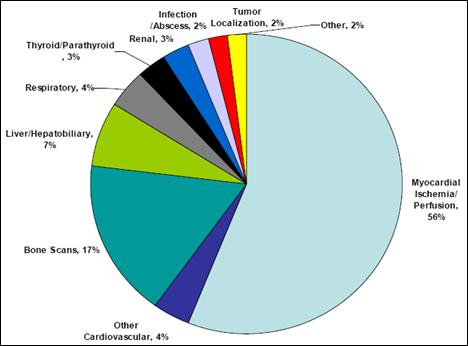
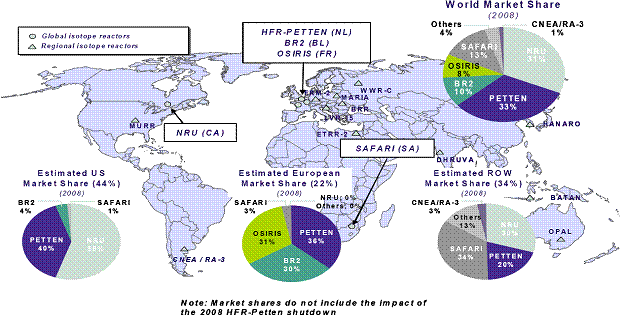
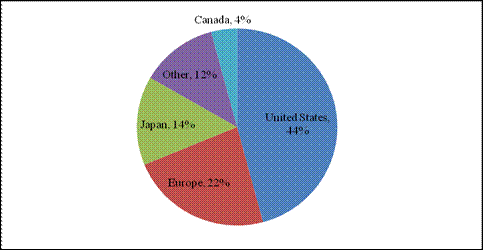
Source: AECL. According to Hugh MacDiarmid, President and Chief Executive Officer of AECL, the unprecedented NRU repair operation was impeded by several technical problems, particularly in the inspection, analysis and understanding of irradiated metal behaviour, and in the measurement and evaluation of stress on the structure of the reactor vessel.[8] Refuelling the reactor required the approval of the CNSC, which, according to William Pilkington, Senior Vice-President and Chief Nuclear Officer of AECL,[9] was facilitated by the Protocol for the Restart of the NRU Reactor whose purpose was to establish the administrative framework, milestones and service standards for the licensing activities related to the restart of the NRU reactor.[10] The 52-year-old NRU reactor had its vessel replaced only once between 1972 and 1974, and the current vessel, according to Mr. Pilkington, "has been inspected several times and has been deemed to be continuing to be fit for service for an extended period into the future".[11] Reactors can be refurbished and maintained with no set lifetime;[12] however, their cost of servicing and vulnerability rises with age.[13] On March 30, 2010, Mr. MacDiarmid informed the Committee that the remainder of the NRU repairs, through the end of July 2010, would cost approximately $44 million in addition to the $72 million already provided by the federal government in the 2009-2010 Supplementary Estimates.[14] The repairs are expected to allow the reactor to operate reliably beyond 2016.[15] The NRU is licensed to operate until October 2011; however, Mr. MacDiarmid indicated that AECL is working with the CNSC to extend the licence to 2016.[16] In order to gain a more thorough understanding of the issues facing the NRU reactor shutdown and repair, the Committee conducted a visit to the Chalk River Facilities on April 13, 2010. During the visit, the members of the Committee had the opportunity to visit the NRU reactor and the Multipurpose Applied Physics Lattice Experiment (MAPLE) reactors. RECOMMENDATION 1: Considering the evidence presented to the Committee regarding the unexpected shutdown of the National Research Universal (NRU) reactor, the Committee recommends that the Government of Canada continue to support the NRU reactor and its return to service, and provide all necessary support, financial and otherwise, to ensure that the NRU reactor licence is renewed until 2016. RECOMMENDATION 2: The Committee also recommends that the Government of Canada instruct Atomic Energy of Canada Limited to produce a report on the shutdown and repair procedures in order to facilitate better planning and faster return to service times in the event of another unexpected shutdown. A. Medical ImplicationsThe unscheduled shutdown of the NRU reactor triggered a global shortage in nuclear medical isotopes (mainly Mo-99), creating a problematic situation particularly from a medical standpoint. Technetium-99m (Tc-99m), which is derived from Mo‑99, is used for about 82% of diagnostic radiopharmaceutical injections-primarily cardiac imaging, bone scans to detect cancers, and general organ scans.[17] When injected into the body, low doses of radioisotopes emit energy that can be captured externally to produce a diagnostic image, hence allowing for an earlier and more complete diagnosis than external imaging procedures. In the case of cancer, radioisotopes are also used therapeutically by emitting energy to target and kill diseased cells.[18] Figure 2 demonstrates the predominance of Tc-99m in the 2006 composition of nuclear medical procedures in Canada. Figure 2-Composition of Nuclear Medical Procedures Source: Natural Resources Canada. The isotope shortage limited diagnostic testing, not therapy, and as a result, affected mostly cancer patients whose early and reliable diagnosis is critical.[19] Following the shutdown, Canada initially experienced an average shortage of about 30%. However, this figure increased to about 60% when the Petten reactor in the Netherlands shut down temporarily in February 2010.[20] The isotope shortage varied across Canada, since isotope supplies are managed by the provinces and territories and because the supply chain of isotopes is different in different parts of the country.[21] According to Dr. Sandy McEwan, Special Advisor on Medical Isotopes to the Minister of Health, Lantheus is a bigger supplier of Tc-99m in the east than in the west, where Covidien handles most of the supply. [22] Dr. McEwan also stated that the areas that appeared to have the most difficulty coping with the shortage were "small urban sites dependant either upon small radiopharmacies or upon a generator being supplied to an individual hospital radiopharmacy".[23] The shortage also resulted in economic losses in some areas. For example, by August 2009, the direct additional costs of medical isotopes to hospitals and clinics in Ontario due to the supply shortage were estimated to be in the order of $1.7 million.[24] According to Dr. McEwan, the situation for patients was of "great concern".[25] As Dr. Eric Turcotte of the Molecular Imaging Centre of Sherbrooke explained, the isotope shortage fluctuated on a daily basis, making it particularly challenging for medical practitioners to schedule radiopharmaceutical exams, even within a 24-hour period. [26] In order to maximize the quantity of technetium for patients, a number of nuclear medicine departments used alternative (and sometimes less effective) medical procedures to compensate for the supply shortage of Tc-99m. They also amended their schedules and were "forced to prioritize [patients], sometimes postponing exams and [...] even restricting the number of patients". [27] Prioritizing patients based on urgency proved to be a difficult task, sometimes involving life-or-death cases. [28] B. Global Supply and DemandFive government-owned reactors supply about 95% of the global demand for Mo‑99: the NRU reactor, the Petten reactor in the Netherlands, the BR2 reactor in Belgium, the OSIRIS reactor in France, and the SAFARI reactor in South Africa. Several other smaller reactors provide isotopes for local and regional use with no major influence on the global supply chain.[29] Figure 3 presents the 2008 global market share of Mo-99m, by reactor. Figure 3-2008 Global Market Share of Mo-99m Source: Government of Canada Response to the Report of the Expert Review Panel on Medical Isotope Production, March 2010. According to the Ad hoc Interservice group of the European
Commission's services on sufficiency in supply of radioisotopes for medical use,
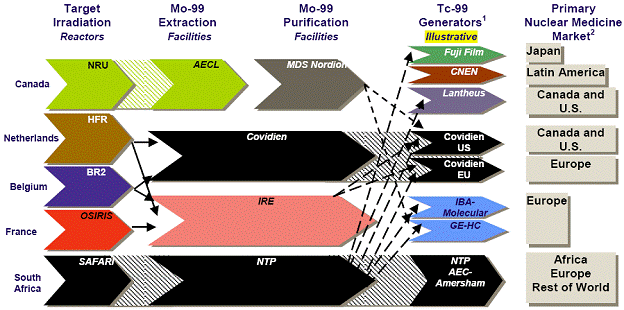
the total number of Figure 4-Approximate Global Demand for Molybdenum-99/Technetium-99m Note: Totals may not sum, due to rounding. Source: Natural Resources Canada. Throughout the supply chain, specific steps must be followed in face of a number of technical and regulatory challenges, which added to the complexity of responding to the global isotope shortage. After being processed at the NRU reactor, Mo-99, which has a half-life[34] of about 66 hours, is shipped to MDS Nordion in Kanata, approximately 23 kilometres southwest of Ottawa, to be extracted and purified. It must then be exported to the appropriate Tc-99m manufacturer in the United States or Japan since there are no manufacturers of Tc‑99m in Canada. Exports to the United States are partly re-imported as Tc-99m generators for medical use in Canada. These generators are produced with a useful life of 10 to 14 days, and, as a result, must be shipped to hospitals and radiopharmacies within an appropriate timeframe. Tc-99m itself has a half-life of only six hours, and therefore cannot be stockpiled. All stages of the supply chain are also subject to nuclear and medical health and safety regulations.[35] Figure 5 demonstrates the global supply chain of Mo‑99/Te‑99m, including the Canadian supply chain. Figure 5-Global Supply Chain of Molybdenum-99/Technetium-99m Source: Expert Review Panel on Isotope Production. Since all reactors must undergo systematic outages for maintenance, there is a critical need for harmonization between isotope suppliers to maintain the global supply balance.[36] Representatives from the global reactors meet quarterly to schedule plant outages and discuss coordination concerns to minimize the impact of scheduled outages on the global supply.[37] According to Robert Atcher, former President of the International Society of Nuclear Medicine (SNM), the NRU reactor "has more excess capacity than the other four reactors," and is therefore capable of making up for a "substantial percentage of the market supplies" whenever one of the other major reactors goes off-line.[38] Furthermore, the NRU design maximizes the production of medical isotopes because it has the ability to yield medical isotopes during scheduled maintenance and can be recharged and refuelled while in operation. As a result, the unexpected and prolonged outage of the NRU reactor caused a serious imbalance within an interdependent supply chain.[39] Future projections indicate that the production of medical isotopes needs to increase, even with the NRU reactor in operation. Tc‑99m is a non-invasive technique with ever-growing applications, and the demand for it is rising worldwide due to the aging populations of Europe and North America and the growing use of medical isotopes in emerging countries.[40] Experts predict a firm annual demand of about 30 million Tc-99m procedures over at least the next 10 years.[41] Some witnesses indicated that the NRU shutdown was potentially threatening the sustainability of Canada's expertise in the field of nuclear medicine, which may compromise the country's future role in the field. On October 19, 2009, Dr. Jean-Luc Urbain, President of the Canadian Association of Nuclear Medicine, told the Committee that "the enrolment of students, mainly technologists and physicians, in nuclear medicine sciences is down [...] and nuclear scientists are contemplating or are already moving out of the country".[42] Kevin Tracey, Vice-President of the Ontario Association of Nuclear Medicine, pointed out that training new technologists is a difficult task, "once they leave the community [...] they go to the U.S., [and getting] them back is an extreme challenge".[43] RECOMMENDATION 3: Considering the important role that Canada plays in the production of medical isotopes, the Committee recommends that the Government of Canada continue to support Canadian involvement in isotope production, especially through the Non-reactor-based Isotope Supply Contribution Program. RECOMMENDATION 4: In the meantime, the Committee recommends that the federal government conduct a cost-benefit analysis of isotope production, and evaluate future production levels of isotopes. RECOMMENDATION 5: Considering the integrated nature of the international isotope supply chain, the Committee also recommends that the federal government continue to support international cooperation and engagement with other isotope suppliers, and continue to improve communication protocols between stakeholders, including the public. RECOMMENDATION 6: Finally, the Committee recommends that the Government of Canada work to ensure continued isotope production in Canada as a measure to strengthen domestic supply and ensure the availability of medical isotopes to patients regardless of changes in international supply. C. Research ImplicationsIn addition to medical isotope production, the NRU reactor is also used for research purposes. According to Dr. Dominic Ryan[44], President of the Canadian Institute for Neutron Scattering, the neutron beams emitted from the reactor core allow for the study of new materials, including "high-Tc superconductors that offer the promise of zero-loss electrical power transport, hydrogen storage materials and battery electrodes that will enable more environmentally friendly uses of power, and high-strength super alloys and composites that will revolutionize manufacturing in the future". This research and development supports Canadian industry by providing "unique knowledge [that] helps companies to develop more competitive products that are safer, more reliable, and less expensive to manufacture". The reactor also contributes to the "stewardship of [Canada's] CANDU fleet and to the development of next-generation reactor designs". According to Dr. Ryan, the extensive research and training supported by the NRU research facilities have "raised Canada's profile as a technology leader around the world".[45] The NRU is not the only nuclear research facility in Canada. For example, the 5 megawatt nuclear reactor at McMaster University is mainly used for research,[46] and the TRIUMF subatomic physics laboratories at the University of British Columbia use accelerator technology to conduct research in particle physics and nuclear physics, including the study of rare isotopes.[47] However, the flexibility of the NRU design has enabled research opportunities in ways not possible at other facilities in Canada.[48] Furthermore, the research role of nuclear research reactors cannot be entirely replaced by alternative research facilities using accelerator technology. According to Dr. Daniel Banks, representative of CREATE (Chalk River Employees Ad-hoc TaskforcE for a national laboratory), the Chalk River and TRIUMF facilities are "complementary [.] rather than redundant" since some of their research functions are not interchangeable. For example, nuclear research reactors can be used to obtain "more precise knowledge of the conditions of materials inside nuclear power reactors that cannot be obtained by other means".[49] According to Dr. Banks, "If for some reason we no longer had the NRU reactor and there [was not] a certainty of a new facility, [.] we would lose critical mass of expertise at Chalk River, probably quite quickly just because most talented scientists and engineers at that facility would be looking for jobs elsewhere".[50] Furthermore, the eventual closure of the NRU reactor may cause a gap for many Canadian and international scientists that use the facilities for research. As Dr. Ryan testified, teams of graduate students and post-docs are brought to the NRU reactor "where they get hands-on training by experts in neutron beam techniques and where they meet researchers from around the world. These are the next generation of Canadian researchers. But if [the] NRU is not replaced, where will they work?" [51] According to Linda Keen, the size of the research gap that could result from the possible closure of the NRU is uncertain at this time.[52] RECOMMENDATION 7: Considering Canada's critical role in medical isotope research, the Committee recommends that the federal government support continued research in medical isotopes. The Expert Panel on Medical Isotope Production maintains that a sustainable supply of Tc-99m should:
Furthermore, the Expert Panel defines a secure supply of Tc-99m as one that would:
The following sections discuss the main alternative medical procedures and supply options identified by the Committee witnesses as existing and potential methods to ensure sustainable and secure future supplies of Mo-99/Tc-99m and alternatives, and to avoid a repeat of the recent isotope shortage. A. Alternative Medical ProceduresNuclear medicine is one of many imaging technologies, and Tc-99m is not the only isotope used for diagnosing cancer-although it typically accounts for about 82% of nuclear medical procedures. Table 2 compares Tc-99m to a list of alternative medical isotopes that could replace Tc-99m in the short term, according to the Ad hoc Interservice group of the European Commission's services on sufficiency in supply of radioisotopes for medical use.[54] Furthermore, there are alternative procedures to Tc-99m imaging, including positron emission tomography (PET) scans, x-ray, computed tomography (CT) scans, magnetic resonance imaging (MRI) scans and ultrasounds.[55] Table 2: Tc-99m and alternative medical isotopes
Source: Adapted from the report of the Ad hoc Interservice group of the European Commission's services on sufficiency in supply of radioisotopes for medical use. The medical community used some alternative procedures as a short-term contingency response to the shutdown of the NRU reactor. However, such alternatives could replace the role of Tc-99m only temporarily, since they are often less effective, available or reliable.[56] Alternative procedures such as CT and MRI scans have limited availability; echocardiography (a potential alternative for cardiac function tests) may not be suitable for 15 to 20% of patients;[57] and thallium emits more radiation than other nuclear medicine and does not yield optimal image quality. The image quality of thallium-based procedures is further worsened if a patient is even slightly overweight, thereby increasing the chance of diagnostic errors.[58] Furthermore, according to the Ad hoc Interservice group of the European Commission's services on sufficiency in supply of radioisotopes for medical use, "no alternative exists today for some [...] Tc-labelled compounds, like colloids (for sentinel lymph node determination for cancer surgery), MAA for lung perfusion, DMSA for kidney function investigation and HAS for gastro-intestinal bleeding or cardiac studies".[59] The isotope supply shortage raised interest in alternative technologies and imaging modalities, particularly PET imaging. PET technology does not need a nuclear reactor because it can use isotopes from cyclotrons that can be located in hospitals and universities. According to Cyrille Villeneuve, Vice President of Lantheus Medical Imaging, the technology offers a good alternative to conventional technologies, "with better sensitivity and specificity".[60] Dr. Éric Turcotte, Medical Specialist in Nuclear Medicine and Clinical Head of the Molecular Imaging Centre of Sherbrooke, supported this view, stating that PET is "by far the best solution for the Canadian public when it comes to diagnosing cancer and other problems".[61] Many experts believe PET to be the "technology of the future".[62] For example, Dr. Nigel Lockyer, Director of TRIUMF, testified that PET imaging is "the fastest-growing component of nuclear medicine," adding that "last year the sales of PET cameras in the U.S. exceeded the sales of SPECT cameras (Single Photon Emission Computed Tomography) [...which] use Mo-99".[63] On the other hand, the technology faces a number of challenges in Canada, including:
According to Mr. Villeneuve, PET technology in Canada "will increase significantly as soon as the equipment and infrastructure are built".[70] Mr. Meyer stated that "it's fairly likely that Canada will move to a network of PET isotope producers" over the next 10 years.[71] In addition to PET scanning, the Canadian Association of Nuclear Medicine has endorsed a new gamma camera, which uses a solid-state crystal detector and resolution recovery software. According to Dr. Urbain, the new camera is an "immediately implementable [short-, medium-, and long-term] solution" that uses two-to-three times less Tc-99m than the traditional SPECT scanners.[72] RECOMMENDATION 8: In light of this evidence, the Committee recommends that, when necessary, the Government of Canada encourage the use of alternative medical isotopes for diagnostics. RECOMMENDATION 9: Furthermore, the Committee recommends that the Government of Canada encourage the development of PET technology considering the pre-clinical and clinical trials performed in Europe and in the United States. B. Potential Supply Options in CanadaIn line with international efforts of nuclear non-proliferation, low enriched uranium (LEU) has become the preferred option over highly enriched uranium (HEU) for use in civil nuclear reactors. LEU has a lower than 20% U-235 concentration, while HEU typically has a U-235 concentration of approximately 93%. The latter, which is also used to produce nuclear weapons, has been the preferred option for isotope production because enriching uranium to over 90% U-235 maximizes the production of Mo-99. Large-scale production of Mo-99 using LEU targets still requires research and development to optimize the process of isotope production and manage the increased volumes of waste.[73] The following sections discuss the main technology options with potential to produce medical isotopes in Canada, as identified by the Expert Panel and by various Committee witnesses. Table 3 summarizes available information for each of the technology options. For a variety of reasons, the Committee did not have the information to conduct a cost-benefit analysis of the capital or final production costs of the various options. Table 3: Summary Information for Each Technology Option
* Capacity to make Mo-99 with a reactor using LEU targets is limited more by the efficiency of the processing facility than by the reactor itself. ** Based on an estimated Canadian demand for Tc-99m of 32,000 doses (470 6-day Ci of bulk Mo-99) per week. *** Based on estimated reactor capacity, without considering limitations of a processing facility. Adapted from the Report of the Expert Review Panel on Medical Isotope Production. New Multi-Purpose Research ReactorThe Expert Panel maintains that building a new multi-purpose research reactor is the option with the "highest potential for concomitant benefit to Canadians based on the promise of the broad-based research that would be undertaken, and its associated potential for generating intellectual property, job creation and training". According to the Expert Panel, with the NRU reactor approaching the end of its life cycle, deciding on a new research reactor "is needed quickly to minimize any gap between the start-up of a new reactor and the permanent shutdown of the NRU".[74] The Expert Panel acknowledges this to be the highest cost solution, pointing out that even though the costs of building a new reactor can be spread over a large spectrum of activities, isotope production would offset only about 10 to 15% of these costs. It was therefore concluded that "building a new reactor would have to be justified, in large part, based on its other missions." The Expert Panel also recommended that any new reactor-based source of Mo-99 should be based on LEU targets, in accordance with international efforts towards nuclear non-proliferation. The use of LEU requires research and development to optimize isotope production and manage the increased volumes of waste.[75] One proposed new research reactor to replace the aging NRU is the Canadian Neutron Beam Centre in Chalk River, which is planned to perform multi-purpose research, and could produce Mo-99 upon conception. Furthermore, according to Mr. Dupont, "the Province of Saskatchewan has indicated it is also interested in discussing with the Government of Canada possible arrangements for a research reactor and, eventually, the production of isotopes".[76] RECOMMENDATION 10: The Committee recommends that the Government of Canada study the feasibility of a new multi-purpose research reactor in order to accurately estimate construction and operating costs as well as potential sources of income and report the results to Parliament. The MAPLE ReactorsThe MAPLE reactors 1 and 2 at AECL's Chalk River laboratories were designed and built exclusively for the production of medical isotopes under contract. They were originally intended to replace the isotope production of the aging NRU reactor, but were never fully commissioned due to a discrepancy between the modelled and actual behaviours of the reactors. According to AECL, the reactor's safety provisions were deemed unsatisfactory since the discrepancy could not be explained unequivocally. Mr. MacDiarmid testified that the discontinued MAPLE reactors cost AECL approximately $250 million,[77] which does not include any costs paid for by MDS Nordion. The reactors are currently in an extended shutdown state.[78] The feasibility of restarting the MAPLE project is controversial. Dr. Jatin Nathwani, Ontario Research Chair in Public Policy for Sustainable Energy Management, Dr. Jean Koclas, Professor at the Nuclear Engineering Institute of the École polytechnique Montréal, and Dr. Daniel Meneley, Acting Dean at the Faculty of Energy Systems and Nuclear Science of the University of Ontario Institute of Technology, expressed support for revisiting the project. Dr. Meneley stated that "the start-up and operation of [the MAPLE] facilities may well be the preferred route [...]" despite their obvious weaknesses.[79] Furthermore, Steve West, President of MDS Nordion, quoted a report by the National Academy of Sciences, stating that AECL could contract with another organization to provide the necessary technical expertise or resources to repair the MAPLE reactors. The report's authoring committee "assumes that the worst-case scenario for fixing the MAPLE reactors involves the replacement of the reactor cores," which would likely cost less than building a new reactor.[80] On the other hand, John Waddington, nuclear safety consultant, indicated that the reactors could be started in principle, but pointed out that this would require "much human and financial effort".[81] Furthermore, Mr. MacDiarmid stated that the MAPLE reactors are not a viable short-term option and would take "many years and many hundreds of millions of dollars" to be licensable and put into service.[82] The Expert Panel maintains that, since the MAPLE reactors were designed to focus on isotope production using HEU, they would pose "significant challenges for possible modification and conversion to LEU". In addition, the Expert Panel states that "even if the existing infrastructure were to come at no cost, the ongoing economics for [the MAPLE] project remain questionable because high operating costs cannot be shared across multiple areas".[83] The MAPLE reactors could not produce all the isotopes produced by the NRU reactor, such as cobalt-60 (also used for cancer treatment) and other isotopes for industrial purposes, and could not be used to perform advanced materials research.[84] RECOMMENDATION 11: If a private sector proposal is made for the MAPLE reactors that accepts fully the commercial risk associated with the reactors and requires no additional costs on the part of the government, the Committee recommends that the Government of Canada remain open to considering the proposal. The McMaster Nuclear ReactorThe McMaster nuclear reactor in Hamilton, Ontario, is a 5-megawatt materials test reactor (MTR). The university has proposed to produce about 20% of North America's Mo‑99 demand in the medium term, which is about the equivalent of four times Canada's demand.[85] The reactor has produced medical isotopes in the 1970s; however, the current supply chain is not set up to incorporate large quantities of isotopes from Hamilton, Ontario. Furthermore, Christopher Heysel, Director of Nuclear Operations and Facilities at the McMaster Nuclear Reactor, indicated that although the proposed isotope production will have the same chemical composition for the targets as the NRU reactor, the pin-shaped NRU target has a different geometry from the McMaster target, which is a pleat design. [86] AECL has indicated its support to the McMaster proposal "to the extent that it is possible;" pointing out that logistical issues associated with transporting radioactive materials across the Greater Toronto and Hamilton Area could pose challenges to implementing this option.[87] The Expert Panel pointed out that using other existing research or power reactors to irradiate targets for the production of Mo-99 requires the use of HEU targets to achieve worthwhile yields, adding that, in accordance with international efforts towards nuclear non-proliferation, HEU-based options should be considered to address only short-term supply shortages. Converting a reactor from HEU (about 93% U-235) to LEU (less than 20% U-235) targets reduces the efficiency of isotope production to about one-fifth for the same amount of uranium and requires structural modifications (e.g. design of new targets). It also incurs other costs as a result of lower yields and increased waste.[88]According to the McMaster University website, "the [McMaster] reactor is currently undergoing a switch from highly-enriched uranium (HEU) to low-enrichment uranium (LEU) fuel[...]"[89] The McMaster reactor, which is licensed until 2014, is currently operating at 3 megawatts, 16 hours a day, five days a week. McMaster's proposal would require that the reactor operation be ramped up to 24 hours a day, seven days a week in order to produce isotopes at the proposed capacity. CyclotronsCyclotrons are particle accelerators whose acceleration path is circular. Although they already produce some medical isotopes (e.g. Thallium-201, Iodine-125 and Gallium‑67), they cannot yet produce Tc-99m for large-scale medical use. According to Tim Meyer, existing cyclotrons have the ability to produce limited amounts of Tc-99m directly by bombarding Mo-100 with protons. Human clinical trials of this method could be conducted within 18 months, and the technology could be deployed without significant changes to the equipment already in place in Canada. On the other hand, cyclotron-produced isotopes have a short half life which limits their use to close-by hospitals and radiopharmacies. However, Mr. Meyer indicated that this is not a major concern since most of Canada's inhabitants are close to major population centres.[90] The Committee is concerned that cyclotrons located only in major population centres would not be easily accessible to some rural communities. The Expert Panel maintains that the early development stage of this technology makes it difficult to determine how much of the Canadian market could be served by cyclotrons. Conversely, this option is attractive because cyclotron infrastructure could offer surge capacity to augment other sources of medical isotopes and still be used for other purposes.[91] It was not clear to the Committee what the total cost of the cyclotron option will be. According to the Expert Panel, the extensive R&D required to develop this option can be done at a low-cost considering the presently available infrastructure to undertake the research, demonstration and initial production. However, the large-scale production and commercialization of cyclotron-produced Tc-99m could have a high cost because of the limited availability of Mo-100, which is currently not produced commercially. [92] Cyclotron-produced Tc-99m may require more validation from a health regulatory perspective than other options. On the other hand, cyclotrons do not produce nuclear waste and are considered the "timeliest option". Depending on R&D results and on health regulatory issues, commercial production could begin between 2011 and 2014. [93] Linear AcceleratorsPhoto-fission OptionThe TRIUMF group at the University of British Columbia (UBC) has proposed an alternative method of producing Mo-99 through nuclear interactions with natural uranium. In the photo-fission option, "a high-power electron linear accelerator is used to bombard a converter to produce an intense photo beam to generate Mo-99 through nuclear interactions with natural uranium".[94] A new multi-purpose research accelerator, known as the e-linac or superconducting electron linear accelerator, is currently under construction at UBC and will be used to validate this option.[95] According to the Expert Panel on Medical Isotope Production, the development of the photo-fission concept requires substantial research and development for the target and converter design, the cooling capacity and overall process optimization. The technology would fit well with the existing supply chain; however, it would generate significant quantities of nuclear waste. Furthermore, to meet the required production levels, the photo-fission accelerators must focus exclusively on isotope production, making for an unfavourable economic case since the technology's relatively high capital investment cannot be shared across multiple missions.[96] Molybdenum-100 Transmutation OptionAn electron linear accelerator can produce Mo-99 through the transmutation of enriched Mo-100, an option proposed by the National Research Council.[97] According to the Expert Panel, this option requires "significant R&D regarding targetry and cooling capacity, as well as the development and marketing of a new type of generator". There is also concern that this technology may not be accepted by hospitals or be able to compete with the traditional generators. [98] According to the Expert Panel, there is currently no commercial production of purified Mo-100, and the quantity needed may entail substantial costs and hence pose a barrier to commercialization. It is unknown whether recycling could reduce these costs because Mo-100 recycling has yet to be demonstrated, which would require significant research and development. Furthermore, this type of accelerator may need to focus exclusively on isotope production to meet the required levels of output, which weakens its economic case, as in the case of the photo-fission option. From an environmental perspective, however, the Panel felt that this option is more favourable than the photo-fission option because it does not generate nuclear waste.[99] RECOMMENDATION 12: The Committee recommends that the federal government learn from the failure of the MAPLE reactors and the impact of the NRU shutdown on medical isotope supplies in Canada, and seek to diversify and secure the supply sources of medical isotopes in the medium and long term by funding several projects out of the $35 million envelope announced in the last federal budget. RECOMMENDATION 13: Furthermore, the Committee recommends that the Government of Canada examine fully all the alternative production proposals, and continue to support the research and development of new technologies. RECOMMENDATION 14: In particular, the Committee recommends that the Government of Canada continue to fund research in accelerator technology, both linear accelerators and cyclotrons. C. Suppliers outside CanadaShortly after the NRU reactor shutdown, the Petten reactor in the Netherlands increased its production by 50% and the SAFARI reactor in South Africa by 20%. Similarly, Belgium expanded its processing capacity to "accommodate larger volumes of irradiation by nuclear reactors," and Australia "intensified efforts to bring its OPAL reactor on stream".[100] Australia has also started producing medical isotopes for its domestic consumption, which freed up supply from the South African reactor for exportation elsewhere.[101] This situation changed following the planned temporary shutdown of the Petten reactor that began in February 2010, which exacerbated the global shortage considerably. Despite international efforts to produce additional isotopes, the global supply of medical isotopes is, at best, unstable. All major reactors that produce medical isotopes are of similar vintage to the NRU (see Table 4) and the sustainability of their isotope production is uncertain. Furthermore, they must all undergo scheduled outages for maintenance.[102] Table 4: The Age of Major Production Reactors
Source: Natural Resources Canada. Other reactors may potentially contribute to the global supply of medical isotopes in the future. These include a reactor in Argentina, which could supply modest quantities to the North American market; the Jules Horowitz reactor in France, which is expected to come on stream by 2015; and the University of Missouri research reactor, which may be brought on stream to produce Mo-99, although there is no specific commitment to do so.[103] RECOMMENDATION 15: Considering the age of the NRU reactor, the Committee recommends that the government recognize that the NRU is not a credible or sustainable long-term solution, but is critical for the short-term supply of isotopes both domestically and globally. The Government of Canada has indicated that it has taken measures to respond to the temporary shutdown of the NRU reactor and the consequential shortage in medical isotopes by:
Budget 2010 also provides $300 million on a cash basis for AECL's operations in 2010-11 to cover "anticipated commercial losses and support the corporation's operations, including the continuous development of the Advanced CANDU Reactor, ensuring a secure supply of medical isotopes and maintaining safe and reliable operations at the Chalk River Laboratories".[106] According to Mr. MacDiarmid, this funding will support a number of activities, "some of which are related to the repair of the NRU and the preparation for the relicensing or the licence extension of the NRU". [107] RECOMMENDATION 16: In addition to these measures, the Committee recommends that the Government of Canada ensure that the provinces and territories are given compensation for the increased costs and additional management costs of technetium-99m incurred by health authorities as a result of the shortage of medical isotopes. RECOMMENDATION 17: Furthermore, the Committee recommends that the Government of Canada issue a public statement to clarify whether or not it intends to get out of the supply side of isotope production by 2016. RECOMMENDATION 18: If the Government of Canada intends to get out of the isotope business as stated by the Prime Minister, the Committee recommends that it issue a public statement and table a detailed exit strategy that includes its plans to keep the NRU reactor operating until 2016. [1] CANDU reactors are Canadian-designed nuclear power reactors that are fuelled by natural uranium and use heavy water as a moderator and coolant. [2] AECL, "Nuclear Science - National Research Universal Profile," n.d., http://www.aecl.ca/Science/CRL/NRU.htm. [3] Dominic Ryan, Canadian Institute for Neutron Scattering, Evidence, June 16, 2009. [4] William Pilkington, AECL, Evidence, June 4, 2009. [5] Michael Binder, CNSC, Evidence, June 4, 2009. [6] Chalk River Information Bulletins, AECL, http://www.aecl.ca/NewsRoom/Community_Bulletins.htm. [7] William Pilkington, AECL, Evidence, October 19, 2009. [8] Hugh MacDiarmid, AECL, Evidence, March 30, 2010. [9] William Pilkington, AECL, Evidence, October 19, 2009. [10] "AECL-CNSC Protocol," AECL: http://www.nrucanada.ca/en/home/projectrestart/aeclcnscprotocol.aspx. [11] William Pilkington, AECL, Evidence, August 21, 2009. [12] Michael Binder, CNSC, Evidence, June 4, 2009. [13] Serge Dupont, Natural Resources Canada, Evidence, June 2, 2009. [14] Hugh MacDiarmid, AECL, Evidence, March 30, 2010. [15] William Pilkington, AECL, Evidence, October 19, 2009. [16] Hugh MacDiarmid, AECL, Evidence, June 4, 2009. [17] Meena Ballantyne, Health Products and Food Branch, Health Canada, Evidence, June 2, 2009; and Cyrille Villeneuve, Lantheus Medical Imaging, Evidence, March 25, 2010. [18] Medical Isotopes, Frequently Asked Questions, March 12, 2008, http://www.medicalisotopes.org/faq.html. [19] Karen Gulenchyn, Department of Nuclear Medicine, Hamilton Health Sciences and St. Joseph's Healthcare Hamilton, Evidence, June 9, 2009. [20] Eric Turcotte, Molecular Imaging Centre of Sherbrooke, as an individual, Evidence, March 25, 2010. [21] Jean-Luc Urbain, Canadian Association of Nuclear Medicine, Evidence, June 9, 2009. [22] Sandy McEwan, Special Advisor on Medical Isotopes to the Minister of Health, as an Individual, Evidence, August 19, 2009. [23] Ibid. [24] Hon. David Caplan, Minister of Health and Long-Term Care, Government of Ontario, Evidence, August 21, 2009. [25] Sandy McEwan, Special Advisor on Medical Isotopes to the Minister of Health, as an Individual, Evidence, August 21, 2009. [26] Eric Turcotte, Molecular Imaging Centre of Sherbrooke, as an individual, Evidence, March 25, 2010. [27] Cyrille Villeneuve, Lantheus Medical Imaging, Evidence, March 25, 2010. [28] Eric Turcotte, Molecular Imaging Centre of Sherbrooke, as an individual, Evidence, March 25, 2010. [29] Serge Dupont, Natural Resources Canada, Evidence, June 2, 2009. [30] Ad hoc Interservice group of the European Commission's services on sufficiency in supply of radioisotopes for medical use, Preliminary Report on Supply of Radioisotopes for Medical Use and Current Development in Nuclear Medicine, October 30, 2009. [31] Serge Dupont, Natural Resources Canada, Evidence, June 2, 2009. [32] AECL, "Nuclear Science-Medical Isotopes," n.d., http://www.aecl.ca/Science/CRL/NRU/Isotopes.htm. [33] Robert Atcher, International Society of Nuclear Medicine, Evidence, August 21, 2009. [34] Half-life is the time it takes for half of a given isotope to undergo radioactive decay. For example, Tc-99m, which has a half-life of six hours, loses half its radioactivity every six hours. [35] Serge Dupont, Natural Resources Canada, Evidence, June 2, 2009. [36] Serge Dupont, Natural Resources Canada, Evidence, June 2, 2009. [37] Richard Côté, AECL, Evidence, October 19, 2009. [38] Robert Atcher, International Society of Nuclear Medicine, Evidence, August 21, 2009. [39] Serge Dupont, Natural Resources Canada, Evidence, June 2, 2009. [40] Jean Koclas, École polytechnique Montréal, Evidence, June 18, 2009. [41] Philippe Hébert, Covidien, Evidence, March 30, 2010; and Cyrille Villeneuve, Lantheus Medical Imaging, Evidence, March 25, 2010. [42] Jean-Luc Urbain, Canadian Association of Nuclear Medicine, Evidence, October 19, 2009. [43] Kevin Tracey, Ontario Association of Nuclear Medicine, Evidence, October 19, 2009. [44] Dominic Ryan, Canadian Institute for Neutron Scattering, Evidence, June 16, 2009. [45] Ibid. [46] Christopher Heysel, McMaster University, Evidence, June 16, 2009. [47] Nigel Lockyer, TRIUMF, Evidence, June 16, 2009. [48] Dominic Ryan, Canadian Institute for Neutron Scattering, Evidence, June 16, 2009. [49] Daniel Banks, as an individual, Evidence, March 25, 2010. [50] Ibid. [51] Dominic Ryan, Canadian Institute for Neutron Scattering, Evidence, June 16, 2009. [52] Linda Keen, as an individual, Evidence, June 16, 2009. [53] Report of the Expert Review Panel on Medical Isotope Production, November 30, 2009, http://www.nrcan.gc.ca/eneene/sources/uranuc/pdf/panrep-rapexp-eng.pdf. [54] Ad hoc Interservice group of the European Commission's services on sufficiency in supply of radioisotopes for medical use, Preliminary Report on Supply of Radioisotopes for Medical Use and Current Development in Nuclear Medicine, October 30, 2009. [55] Meena Ballantyne, Health Products and Food Branch, Health Canada, Evidence, June 2, 2009. [56] Meena Ballantyne, Health Products and Food Branch, Health Canada, Evidence, June 2, 2009. [57] Karen Gulenchyn, Department of Nuclear Medicine, Hamilton Health Sciences and St. Joseph's Healthcare Hamilton, Evidence, June 9, 2009. [58] Eric Turcotte, Molecular Imaging Centre of Sherbrooke, as an individual, Evidence, March 25, 2010. [59] Ad hoc Interservice group of the European Commission's services on sufficiency in supply of radioisotopes for medical use, Preliminary Report on Supply of Radioisotopes for Medical Use and Current Development in Nuclear Medicine, October 30, 2009. [60] Cyrille Villeneuve, Lantheus Medical Imaging, Evidence, March 25, 2010. [61] Eric Turcotte, Molecular Imaging Centre of Sherbrooke, as an individual, Evidence, March 25, 2010. [62] Ibid. [63] Nigel Lockyer, TRIUMF, Evidence, June 16, 2009. [64] Eric Turcotte, Molecular Imaging Centre of Sherbrooke, as an individual, Evidence, March 25, 2010. [65] Jean-Luc Urbain, Canadian Association of Nuclear Medicine, Evidence, October 19, 2009. [66] Eric Turcotte, Molecular Imaging Centre of Sherbrooke, as an individual, Evidence, March 25, 2010. [67] Meena Ballantyne, Health Products and Food Branch, Health Canada, Evidence, June 2, 2009. [68] Karen Gulenchyn, Department of Nuclear Medicine, Hamilton Health Sciences and St. Joseph's Healthcare Hamilton, Evidence, June 9, 2009. [69] Tim Meyer, TRIUMF, Evidence, March 25, 2010. [70] Cyrille Villeneuve, Lantheus Medical Imaging, Evidence, March 25, 2010. [71] Tim Meyer, TRIUMF, Evidence, March 25, 2010. [72] Jean-Luc Urbain, Canadian Association of Nuclear Medicine, Evidence, October 19, 2009. [73] Report of the Expert Review Panel on Medical Isotope Production, November 30, 2009, http://www.nrcan.gc.ca/eneene/sources/uranuc/pdf/panrep-rapexp-eng.pdf. [74] Report of the Expert Review Panel on Medical Isotope Production, November 30, 2009, http://www.nrcan.gc.ca/eneene/sources/uranuc/pdf/panrep-rapexp-eng.pdf. [75] Ibid. [76] Serge Dupont, Natural Resources Canada, Evidence, June 2, 2009. [77] Hugh MacDiarmid, AECL, Evidence, June 4, 2009. [78] Ibid. [79] Jean Koclas, École polytechnique Montréal; Daniel Meneley, University of Ontario Institute of Technology; and Jatin Nathwani, University of Waterloo, Evidence, June 18, 2009. [80] Steve West, MDS Nordion, Evidence, June 11, 2009. [81] John Waddington, Nuclear Safety Consultant, Evidence, June 11, 2009. [82] Hugh MacDiarmid, AECL, Evidence, June 4, 2009. [83] Report of the Expert Review Panel on Medical Isotope Production, November 30, 2009, http://www.nrcan.gc.ca/eneene/sources/uranuc/pdf/panrep-rapexp-eng.pdf. [84] Daniel Banks, as an individual, Evidence, March 25, 2010. [85] Christopher Heysel, Director of Nuclear Operations and Facilities, McMaster Nuclear Reactor, McMaster University, Evidence, June 16, 2009. [86] Ibid. [87] Hugh MacDiarmid, AECL, Evidence, August 21, 2009. [88] Report of the Expert Review Panel on Medical Isotope Production, November 30, 2009, http://www.nrcan.gc.ca/eneene/sources/uranuc/pdf/panrep-rapexp-eng.pdf. [89] McMaster University, Nuclear Engineering and Science, http://engphys.mcmaster.ca/research/areas/nuc.htm [90] Tim Meyer, TRIUMF, Evidence, March 25, 2010. [91] Report of the Expert Review Panel on Medical Isotope Production, November 30, 2009, http://www.nrcan.gc.ca/eneene/sources/uranuc/pdf/panrep-rapexp-eng.pdf. [92] Ibid. [93] Ibid. [94] Ibid. [95] Tim Meyer, TRIUMF, Evidence, March 25, 2010. [96] Report of the Expert Review Panel on Medical Isotope Production, November 30, 2009, http://www.nrcan.gc.ca/eneene/sources/uranuc/pdf/panrep-rapexp-eng.pdf. [97] Nigel Lockyer, TRIUMF, Evidence, June 16, 2009. [98] Report of the Expert Review Panel on Medical Isotope Production, November 30, 2009, http://www.nrcan.gc.ca/eneene/sources/uranuc/pdf/panrep-rapexp-eng.pdf. [99] Ibid. [100] Serge Dupont, Natural Resources Canada, Evidence, August 21, 2009. [101] Richard Côté, AECL, Evidence, October 19, 2009. [102] Steve West, MDS Nordion, Evidence, June 11, 2009. [103] Serge Dupont, Natural Resources Canada, Evidence, June 2, 2009. [104] Government of Canada Response to the Report of the Expert Review Panel on Medical Isotope Production, March 31, 2010. [106] Government of Canada Response to the Report of the Expert Review Panel on Medical Isotope Production, March 31, 2010. [107] According to AECL, the majority of the $300 is earmarked for the "unexpected financial requirements associated with the completion of the life-extension projects at Bruce Power and Point Lepreau." See Hugh MacDiarmid, AECL, Evidence, March 30, 2010. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||