HESA Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
Towards Open Science: Promoting Innovation in Pharmaceutical Research and Development and Access to Affordable Medications Both in Canada and Abroad
Introduction
The rising costs of new drugs have raised concerns both in Canada and worldwide about access to such drugs; and the strain that they place on health care budgets.[1] Furthermore, the role of publicly funded research in the development of innovative drugs has led to “increasing scrutiny of whether adequate conditions are placed on public research and development (R&D) funding to ensure that medicines and other health technologies are affordable to the very public who have financed their development.”[2] It is within this context that the House of Commons adopted Private Members’ Business M-132 on 8 November 2017, which states:
That the Standing Committee on Health be instructed to undertake a study on ways of increasing benefits to the public resulting from federally funded health research, with the goals of lowering drug costs and increasing access to medicines, both in Canada and globally; and that the Committee report its findings and recommendations to the House no later than one year from the time this motion is adopted.[3]
On 16 and 18 October 2018, the House of Commons Standing Committee on Health (the Committee) held two meetings as part of this study, where it heard from a range of witnesses including health researchers; health research funding organizations; patient groups and civil society organizations. The Committee also received 23 written submissions and 7 background documents that further informed the study. Drawing on witness testimony and written submissions, this report examines the role the federal government can play in fostering pharmaceutical research and development both in Canada and globally to ensure that pharmaceutical drugs are accessible and affordable. It begins by providing an overview of the key challenges facing the current model of pharmaceutical research and development. It then examines how the federal government can address some of these challenges by increasing public funding for health research, focusing specifically on translational biomedical research including funding for clinical trials; fostering the development of innovative models of drug discovery; and establishing strategic priorities for drug research and development in line with both domestic and international population health needs.
Overview of the challenges facing pharmaceutical research and development both in Canada and Abroad
Traditionally, drugs are developed by a for-profit pharmaceutical organization that conducts the steps in the R&D process (see Figure 1).[4] The discovery phase of drug development involves the identification and validation of a disease-relevant biological “target,” such as a protein or gene, that can be targeted by a molecule that has the potential to be turned into a drug.[5] A chemical compound is then developed to act on the biological target. The safety and efficacy of the candidate drug is then tested in preclinical and clinical trials. If the trials are successful, the drug is registered with regulatory authorities to seek market authorization. The pharmaceutical company then manufactures the drug and promotes its sale through marketing.
Intellectual property rights in the form of patent and data protection prevent others from making, using or selling the drug for a specified period of time, except when under license, allowing drug manufacturers to maintain a monopoly position in the market.[6] In Canada, under section 44 of the Patent Act,[7] pharmaceutical companies may apply for 20 years of exclusivity for an invention. However, manufacturers of generic pharmaceuticals may challenge the patent prior to its expiry under the Patented Medicines (Notice of Compliance) Regulations (SOR/93-133).[8] Nonetheless, the Food and Drug Regulations grant “innovative drugs” as defined under section C.08.004.1(1), a period of data protection of eight years[9], which prevents generic drugs from making use of the safety and efficacy data of the original drug manufacturer to receive regulatory approval for sale from Health Canada (section C.08.004.1(3)).[10] This provision allows manufacturers of innovative drugs to have a period of market exclusivity of eight years.
Publicly funded research institutions such as universities and government-run labs also participate in the pharmaceutical development process. Scientists working in universities and government labs are also engaging in research in the discovery and preclinical drug development phases and then academic institutions or governments are licensing the intellectual property rights arising from these discoveries to pharmaceutical companies for further product development, including clinical trials and commercialization, in exchange for royalties.[11]
Figure 1—Overview of the Pharmaceutical Drug Research and Development Process

Source: Figure prepared by the Library of Parliament using data obtained from Chas Bountra et al., A New Pharmaceutical Commons: Transforming Drug Discovery, Oxford Martin Policy Paper, 2017.
The Committee heard from some witnesses that this traditional process of drug R&D by pharmaceutical manufacturers is no longer working.[12] The witnesses suggested that it results in the development of costly medicines that place significant financial pressure on public health care systems in countries around the world. For example, Ms. Louise Kyle, North American Coordinating Committee Member, Universities Allied for Essential Medicines (UAEM) explained to the Committee that Sofosbuvir is a drug that can cure over 90% of hepatitis C cases.[13] It was developed over a 10-year period through public funding provided by the United States (U.S.) Department of Veterans Affairs and U.S. National Institutes of Health to Emory University.[14] The drug was commercialized by Pharmasset, which was subsequently acquired by Gilead Sciences. The drug now costs $1000 per pill with the full cost of treatment amounting to $68,714 CAD.[15] According to the Patented Medicine Prices Review Board, Sufosbuvir and other new drugs for the treatment of hepatitis C accounted for an 8% increase in costs of public prescription drug coverage programs in Canada in 2015-2016.[16] Dr. Jason Nickerson, Humanitarian Affairs Advisor, Doctors Without Borders, explained to the Committee that pharmaceutical companies justify the high prices charged for medications based upon the R&D costs of those drugs.[17] Pharmaceutical companies cite research by Tufts Centre for Drug Development, which has estimated the cost of bringing a new successful therapy to market at US $2.6 billion.[18] However, Dr. Nickerson explained that there is no transparency regarding the amount invested by pharmaceutical companies in R&D, therefore it is difficult to determine whether these estimates are accurate and/or the extent to which public funds have contributed to the R&D process like in the case of Sofosbuvir.[19] Furthermore, he noted that alternative not-for-profit pharmaceutical companies have shown that the R&D costs for a new chemical entity can be lower, between C$144 million and C$216 million.[20]
Globally, despite literally trillions of dollars of public funding over the past decades, and an equal amount of private sector funding, we are inventing too few medicines. What’s worse, those medicines we invent are priced at levels that will cripple our health care system and are unaffordable to most people on the planet. Something is not right, obviously.
Dr. Aled Edwards, Chief Executive Officer, Structural Genomics Consortium
The Committee also heard that pharmaceutical companies both in Canada and globally are not focusing their investments on developing drugs and treatments to meet urgent population health needs.[21] For example, Ms. Rachel Kiddell-Monroe, Board Member, Universities Allied for Essential Medicines (UAEM), explained that pharmaceutical companies are not focusing their efforts on developing better and more affordable treatments for tuberculosis:
Even here in Canada, we're watching as our Inuit populations are suffering from 300 times the rate of tuberculosis over and above that of the non-indigenous Canadian born population.
This national crisis that we have here in Canada today, around tuberculosis, is also reflecting a global crisis that we have around tuberculosis. A global crisis which is killing two million people per year; people with multi-drug resistant tuberculosis today are dying because they do not have access to the treatment that they need.
The treatment that exists is over 63 years old; 14,000 pills and multiple injections which leave one in two people deaf. This is the treatment that most people with multi-drug resistant TB are using today. There is a new drug, an amazing new drug which could really change things, but it's just too expensive and it's out of reach for most of those people.[22]
Dr. Jason Nickerson further articulated:
To add to that, we have a major problem with tuberculosis drug development. We are quite simply running out of viable options, and this is a global problem. The Canadian tuberculosis standards reflect global treatment options that are available to everyone, and the options are quite limited. This is a disease for which there is a growing resistance to the drugs that we have available.
In the last 40-plus years, two drugs have entered the market for a disease for which there are 10 million new cases and close to two million deaths per year—two drugs since 1971. Neither is registered in Canada.[23]
The Committee also heard from Dr. Aled Edwards, Chief Executive Officer, Structural Genomics Consortium, that the current model of pharmaceutical R&D through its prioritization of secrecy, patents and private funding inhibits the development of innovative treatments to address current health challenges, such as Alzheimer’s disease,.[24] Dr. Aled Edwards suggested that secrecy among pharmaceutical companies creates redundancy in research, which both increases costs and exposes clinical trial participants to unnecessary health risks:
The Alzheimer’s example is a great one. The beta amyloid hypothesis has been tested by a about 10 companies. Probably about $20 billion has gone into that hypothesis. All the companies did it in secret. We’re still no wiser as to whether that hypothesis is true or not for Alzheimer’s. It was a tragic waste of money. If one had imagined a different universe where we tested that hypothesis once or twice in the open, then 10 people wouldn’t have had to spend $2 billion each and we would have come up with the answer transparently.[25]
Furthermore, he explained that the current approach to pharmaceutical R&D ignores more promising areas of research because they are not financially lucrative or are considered too risky.[26]
In addition, the Committee heard from witnesses that there is actually very limited pharmaceutical R&D being conducted in Canada[27], even though Canadian prices for both generic and patented drugs are 30% higher than the average among Organisation for Economic Co-operation and Development countries, excluding the United States.[28] Moreover, in return for the increased patent protection provided by the 1987 amendments to the Patent Act, Canada's brand name pharmaceutical industry made a commitment that it would increase its annual R&D expenditures as a percentage of sales to 10%.[29]. However, the Committee heard that annual R&D expenditures by brand name pharmaceutical companies as a percentage of sales to R&D in the country has declined steadily since 2003, amounting to 4.1% in 2017.[30] As Dr. Aled Edwards noted, “the global research and development sites, there used to be some in Canada, there are very few anymore.”[31]
Finally, witnesses explained that Canadian universities are also not benefitting from licensing of intellectual property arising from their biomedical research to pharmaceutical companies, because the laboratory discoveries are occurring too early in the drug development process to obtain a return on investment.[32] Consequently, universities do not make significant royalties on licensing their intellectual property to pharmaceutical companies, as Dr. Aled Edwards noted to the Committee:
If you just do a financial, and how much we gain and lose at our universities, we lose money on our intellectual property portfolio. The intellectual property we invent is so early it’s not a product yet […] It wouldn’t be such a bad deal if we got out of the business of trying to pretend we’re little companies at universities.[33]
How the federal government could foster innovation in pharmaceutical research and development in Canada and globally
Witnesses suggested to the Committee that the federal government could address these challenges by increasing its investments in health research; promoting the creation of innovative alternative models of pharmaceutical R&D; and establishing strategic priorities for pharmaceutical R&D in line with both domestic and international population health needs.
A. Federal Funding for Health Research
The Canadian Institutes of Health Research (CIHR) is the main federal research granting agency that supports health research in Canada. In 2017-2018, it provided $1 billion in health research funding, 46% of which ($472 million) supported biomedical research.[34] The Committee heard from Dr. Salim Yusuf, Distinguished University Professor of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences that more federal investment in health research through CIHR is necessary to promote innovation in pharmaceutical R&D in Canada. He explained that innovation in the pharmaceutical sector results from research across the spectrum from basic science, population health science to drug laboratory discoveries made by industry.[35] For example, the Committee heard that blood pressure medications to help reduce the incidence of strokes and heart attacks would not have been developed by industry had there not been initial population health research that found that individuals with higher blood pressure had more strokes.[36] Furthermore, Dr. Salim Yusuf stressed that it is also important to fund translational research, such as clinical trials, to determine whether discoveries in the laboratory are safe for human use and can be translated into health care systems.
The Committee also heard from Dr. Salim Yusuf that Canada’s investments in biomedical research across the spectrum are low in comparison to other industrialized countries. In 2012, Canada spent US$5.3 billion on health research with US$3.3 billion originating from public sources and US$2.0 billion originating from industry, which is below health research investments made by the United States, Western Europe, Japan, Australia and South Korea.[37] In terms of federal funding of health research on a per capita basis and as a proportion of Gross Domestic Product, the Committee heard that CIHR funding is approximately “one-fourth the U.S. and one-half of the U.K. So relative to the size of our economy, relative to our population, we are underfunded from public sources.” (See Figure 2.)[38]
Figure 2—Federal Funding for Health Research per capita and percentage of Gross Domestic Product, 2012
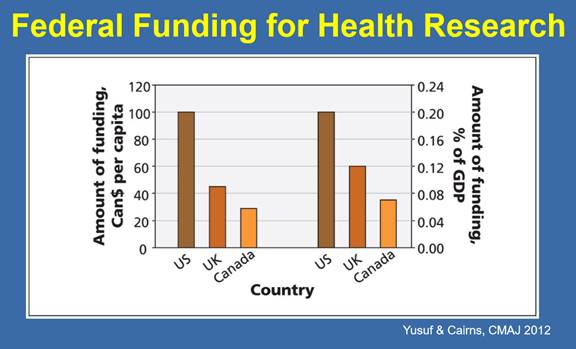
Source: Dr. Salim Yusuf, Distinguished University Professor of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Biomedical Research in Canada, written submission to HESA, October 2018.
Most critically, Dr. Salim Yusuf suggested that there is an imbalance in federal funding between basic biomedical research and clinical trials. He explained that 3.3% of CIHR’s budget supports clinical trials, in comparison to 11% of budget of the U.S. National Institutes of Health and between 20%-25% of the U.K. National Institute for Health Research’s budget.[39] The Committee heard that a lack of public funding for clinical trials means that Canadian researchers face challenges in translating their discoveries into practice, and/or the clinical trials need to be conducted by industry:
Right now to get a clinical trial started after you get funding, you have about 100 separate steps. For a large clinical trial, those steps cumulatively add up to about a million dollars. For an academic investigator to do that, it’s difficult. For industry, they have the resources and the manpower to do that.[40]
Consequently, witnesses recommended the creation of a specific federal funding mechanism through CIHR to support clinical trial research in Canada, which could be leveraged with funding from the pharmaceutical industry at a ratio of 2:1, where the federal government invests $1 for every $2 dollars invested by industry.[41] In its written submission to the Committee, CIHR highlighted that it is providing some support to bridge the gap between discovery research and early stage clinical trials through the Networks of Centres of Excellence program. For example, this program provides funding to organizations such as Accel-RX Health Sciences Accelerator, which in turn provides capital and expertise to support promising drug discovery projects from academia and biotech companies in validating drug targets and identifying drug candidates for clinical trials.[42]
Individual investigators might have good ideas, but to do a 10,000 person clinical trial is unrealistic. Some support mechanisms to perform those trials would reduce barriers for sure.
Dr. Keith Fowke, Professor, Department of Medical Microbiology and Infectious Diseases, University of Manitoba
B. Promoting the Development of Innovative Models of Pharmaceutical Research and Development
I don’t know, I wish there was a better answer and I wish there was a pharmaceutical company that sort of had a good heart.
Louise Kyle, North American Coordinating Committee Member, Universities Allied for Essential Medicines
While witnesses agreed that increases in federal health research funding are necessary to foster innovation in drug R&D in Canada, the Committee also heard that the federal government needs to support the development of alternative models of pharmaceutical R&D that would also prioritize affordable access to drugs. Witnesses appearing before the Committee outlined alternative approaches towards pharmaceutical R&D and highlighted ways the federal government could support the development of these models.
(i) “Open Science” Models of Pharmaceutical Research and Development
The Committee heard that “open science” models of biomedical research are public‑private partnerships among academia, pharmaceutical companies, governments, non‑governmental organizations, and health charities that collaborate in discovery and development of drugs.[43] Within these partnerships, resources, capabilities, financing and intellectual property in both the public and private sector are pooled to support the drug R&D process. However, the science developed through these partnerships remains open in the public domain and is accessible to the research community and is not patented.[44] In addition, the public-private partnership is based upon the agreement that commercial drug products that arise from this collaboration will be priced affordably. This goal can be achieved through licensing agreements, in which a pharmaceutical company is granted rights to manufacture and sell a final product but agrees to sell it at an affordable price because it did not bear the full cost or financial risks associated with developing the drug.[45] Alternatively, the public-private partnership can enter into a non‑exclusive licensing agreement with a pharmaceutical manufacturer, which allows for more than one manufacturer to produce the product, creating competition and driving the price for the drug down.[46]
Figure 3– Open Science Models of Pharmaceutical Research and Development
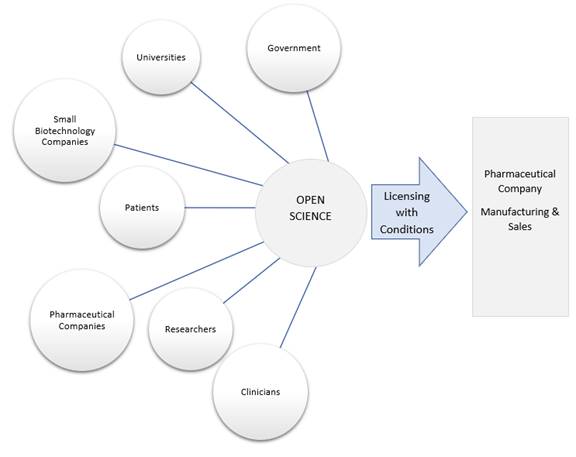
Source: prepared by Library of Parliament
The Committee heard from Dr. Aled Edwards that the Structural Genomics Consortium, a global public-private partnership with the pharmaceutical industry that follows an open science model of biomedical research, had been successful in attracting a $200 million investment from pharmaceutical companies and accelerating downstream drug discovery.[47] Because of the success of this initiative, Dr. Edwards founded M4K Pharma Inc. in 2017, an open science drug company that develops medicines for rare paediatric diseases. Through funding and contributions from Genome Canada, the Ontario Institute for Cancer Research, and pharmaceutical companies, M4K Pharma has developed a candidate drug for a deadly childhood brain cancer called diffuse intrinsic pontine glioma.
Dr. Jason Nickerson explained to the Committee that Doctors without Borders had developed a similar initiative in collaboration with five public research institutions, called the Drugs for Neglected Diseases initiative (DNDi).[48] It is a not-for-profit product development partnership that develops treatments for diseases in neglected populations. Through public and private funding, DNDi has developed seven new treatments for five diseases (malaria, sleeping sickness, viral leischmaniasis, Chagas disease and paediatric HIV) at a cost of C$375 million. DNDi then licenses its discoveries to pharmaceutical companies and places conditions on accessibility and affordability in the countries and populations where the diseases are endemic. Royalties from sales are used to fund further research.
Witnesses appearing before the Committee highlighted ways the federal government could promote the development of open science models of pharmaceutical R&D. Representatives of UAEM recommended that CIHR make “Global Access Licensing” a requirement for obtaining health research funding.[49] A Global Access Licensing requirement would mean that recipients of federally funded health research, including individuals and universities, who choose to commercialize their research must include affordability and access requirements in their licensing agreements with manufacturers and/or negotiate non-exclusive licensing agreements, which will promote competition among manufacturers. The Committee heard that Global Access Licensing had been adopted by the University of British Columbia, Yale University, Johns Hopkins University and Harvard University, as well as other leading academic institutions in Europe.[50]
In their written submission to the Committee, Dr. Edwards and Max Morgan from the Structural Genomics Consortium recommended that CIHR’s existing research funding programs be adjusted to allow for applications from public-private partnerships that follow an open model of drug discovery that is not based upon intellectual property-driven business models.[51] In its appearance before the Committee, Genome Canada recommended that the federal government renew its five-year contribution agreement of $630 million to the organization so that it could continue to provide support to initiatives such as the Structural Genomics Consortium.[52]
Dr. Edwards also recommended that Health Canada amend the Food and Drug Regulations to allow for a special designation for drugs developed through an open science model of drug discovery on the condition that companies agree to an affordable price for their innovative drugs and commit to maintaining open access to their data.[53] This special designation could provide open science drug development companies with incentives such as fast-track approvals and extended periods of market exclusivity and data protection under the regulations without the need for patents. Finally, Dr. Karen Lee, National Vice-President, Research, Multiple Sclerosis Society of Canada recommended that the federal government develop a framework to promote coordination and leveraging of health research funds among governments, universities, private industry and health charities to support the development of innovative treatments.[54]
(ii) Repurposing of Existing Drugs
Dr. Keith Fowke, Professor, Department of Medical Microbiology and Infectious Diseases, University of Manitoba, told the Committee that federal funding of research focusing on examining ways to repurpose existing drugs that are safe, affordable and globally available to treat new conditions is another possible approach of reducing the costs of drug development, while ensuring affordable access to treatments.[55] He explained to the Committee that his CIHR-funded research on HIV/AIDS examined the role that aspirin could play in preventing the spread of infection by reducing inflammation in cells in the genital tract that are susceptible to the HIV virus. His research showed that aspirin reduced the number of HIV target cells in the genital tract by 35%, which is paving the way for clinical trials in this area. Dr. Fowke recommended that CIHR continue to support innovative fundamental research that focuses on the repurposing of existing widely available generic drugs for the treatment of new conditions, an approach that reduces timelines and costs for R&D as these medications already exist. According to Dr. Jason Nickerson, DNDi had also focused its research efforts on the repurposing of existing drugs for the treatment of new conditions, which cost the organization between C$14 million to C$58 million to develop.
(iii) Public Manufacturing of Essential Medicines
The Committee also heard that Brazil and India have successfully ensured affordable access to essential medicines by publicly manufacturing generic drugs.[56] Dr. Salim Yusuf underscored that this approach could be employed to reduce Canadian drug costs in the long-term since generic drugs in Canada are currently “five to 10 times more costly than those in the U.S. and several times more than those in the U.K.”[57]
C. Develop a Strategic Framework for Federally Funded Health Research in Canada and Abroad
Finally, the Committee heard from both Drs. Nickerson and Yusuf that the federal government needs to develop a strategic framework that identifies priorities for health research funding that focuses on population health needs both in Canada and abroad.[58] Though various federal government departments and agencies, including CIHR, the Public Health Agency of Canada and Global Affairs Canada have proposed priorities for federal health research funding both nationally and internationally, witnesses indicated that there is a need to review and better coordinate health research priorities across government to determine whether they are meeting population health needs. This priority setting would help identify areas where the federal government should target its investments in pharmaceutical R&D, which would leverage Canadian expertise across governments, universities, industry and civil society.[59]
Committee Observations and Recommendations
The Committee’s study highlighted that more public investment in health research is necessary to promote innovation in pharmaceutical R&D in Canada. Witnesses suggested that increasing federal investments in health research across the continuum from fundamental research to population health research and clinical trials would support the development of new ideas that would lead to the discovery of new treatments for diseases. More specifically, the Committee heard that targeted federal investments through CIHR are needed in the area of clinical trials to bridge the gap between discovery research and translation of these discoveries into pharmaceutical drugs that are safe and effective in human populations. However, the Committee also heard that it is necessary to ensure that federal funding in pharmaceutical R&D also results in the creation of drugs and other health technologies that are affordable. To that end, witnesses recommended that recipients of federally funded health research be required to include affordability and access requirements in their licensing agreements with drug manufacturers and/or negotiate non-exclusive licensing agreements. They also recommended that the federal government, through regulatory incentives and other measures, foster the development of alternative models of pharmaceutical R&D that prioritize open access to research findings and affordable medicines. Finally, witnesses suggested that it is necessary for the federal government to establish priorities for new innovative models of pharmaceutical R&D and partnerships among governments, health charities, universities and private industry to leverage funding and expertise in these areas. The Committee agrees with the views of witnesses and therefore recommends:
Recommendation 1
That the Government of Canada create a specific funding mechanism for the development of clinical trial research and infrastructure in Canada through the Canadian Institutes of Health Research.
Recommendation 2
That the Government of Canada increase its funding for clinical trial research and infrastructure in Canada to 10% of the Canadian Institutes of Health Research’s budget to be on par with jurisdictions leading in this area, such as the United Kingdom and the United States.
Recommendation 3
That the Government of Canada explore ways to incentivize clinical trial research in Canada for pharmaceutical drugs and incentivize and support the production of those drugs in Canada at an advantaged price for Canada and provide venture capital for the proponent.
Recommendation 4
That the Canadian Institutes of Health Research attach a Global Access Licensing requirement to recipients of its research funding that wish to commercialize their research findings.
Recommendation 5
That the Canadian Institutes of Health Research include in its existing research and development programs support for the development of open science models of drug discovery.
Recommendation 6
That the Canadian Institutes of Health Research develop a framework for open science that supports collaboration and the leveraging of research funding among different partners in pharmaceutical research and development, including health charities, universities, governments, and private industry.
Recommendation 7
That Health Canada develop regulatory incentives for pharmaceutical companies that commit to open access to their research data and affordable prices for their products.
Recommendation 8
That the Government of Canada undertake a strategic review of its health-related research funding priorities across departments and agencies to enhance coordination, including Health Canada, Public Health Agency of Canada, Canadian Institutes of Health Research, Global Affairs Canada, and Innovation, Science and Economic Development Canada.
Recommendation 9
That the Government of Canada explore the feasibility of the public manufacturing of generic medicines.
[1] Suerie Moon, PhD, Director of Research, Global Health Centre & Visiting Lecturer, Interdisciplinary Programmes, Graduate Institute of International and Development Studies, Geneva, Written submission to House of Commons Standing Committee on Health (HESA), 18 October 2018.
[2] Ibid.
[3] House of Commons, Parliament of Canada, Journals, 1st Session, 42nd Parliament, 8 November 2017.
[4] Chas Bountra et al, A New Pharmaceutical Commons: Transforming Drug Discovery, Oxford Martin Policy Paper, 2017.
[5] Daniel L. Shaw, “Is Open Science the Future of Drug Development?,” in Yale Journal of Biology and Medicine, March 2017, vol. 90, no. 1, pp. 147–151.
[6] University College London, “The people’s prescription: Re-imagining health innovation to deliver public value,” Background document submitted to HESA, October 2018.
[7] Patent Act (R.S.C., 1985, c. P-4).
[9] This is subject to change once the United States-Mexico Canada Agreement comes into force, which extends data protection to 10 years for biologic drugs only. Government of Canada, Summary Backgrounder: United States-Mexico-Canada Agreement (USMCA).
[10] Food and Drug Regulations (C.R.C., c. 870) and Health Canada, Guidance Document: Data Protection under C.08.004.1 of the Food and Drug Regulations.
[11] Doctors without Borders, Submission to the Standing Committee on Health Study on Federally Funded Health Research (M-132), October 2018.
[12] HESA, Evidence, 1st Session, 42nd Parliament, 16 October 2018, 0920 (Dr. Aled Edwards, Chief Executive Officer, Structural Genomics Consortium) and HESA, 1st Session, 42nd Parliament, 18 October 2018, (Louise Kyle, North American Coordinating Committee Member and Rachel Kiddell-Monroe, Board Member, Universities Allied for Essential Medicines (UAEM), and Dr. Jason Nickerson, Humanitarian Affairs Advisor, Doctors without Borders).
[13] House of Commons Standing Committee on Health (HESA), Evidence, 1st Session, 42nd Parliament, 18 October 2018, 0905 (Kyle).
[14] University College London, “The people’s prescription: Re-imagining health innovation to deliver public value,” Background document submitted to HESA, October 2018.
[15] Patented Medicine Prices Review Board, “CompassRx, 4th Edition-Annual Public Drug Plan Expenditure Report, 2016/17,” September 2018 and HESA, Evidence, 1st Session, 42nd Parliament, 18 October 2018, 0905 (Kyle).
[16] Ibid.
[18] University College London, “The people’s prescription: Re-imagining health innovation to deliver public value,” Background document submitted to HESA, October 2018.
[20] Ibid.
[21] Ibid., 0900 (Kiddell-Monroe) and 0855 (Nickerson).
[22] Ibid., 0900, (Kiddell-Monroe).
[23] Ibid., 0940 (Nickerson).
[24] Structural Genomics Consortium, “Standing Committee on Health Study M-132 Structural Genomics Consortium Briefing Note: Specific Policy Recommendations,” 17 October 2018.
[26] Structural Genomics Consortium, “Standing Committee on Health Study M-132 Structural Genomics Consortium Briefing Note: Specific Policy Recommendations,” 17 October 2018.
[27] HESA, Evidence, 16 October 2018, 1005 (Dr. Salim Yusuf, Distinguished University Professor of Medicine, Population Health Research, McMaster University and Hamilton Health Sciences, as an Individual and Dr. Aled Edwards).
[28] Universities for Essential Medicines (UAEM), Submission to the Standing Committee on Health for their Study on increasing the benefits resulting from federally funded research, with the goals of lowering drug costs and increasing access to medicines, both in Canada and abroad (M-132), October 2018.
[29] Doctors without Borders, Submission to the Standing Committee on Health Study on Federally Funded Health Research (M-132), October 2018.
[30] Mariana Mazzucato, Professor in the Economics of Innovation and Public Value, University College London, “Submission to the Standing Committee on Health Study on Federally Funded Health Research (M-132),” October 2018.
[33] Ibid., 1005 (Edwards).
[34] Canadian Institutes of Health Research (CIHR), CIHR in Numbers, 2017-18.
[36] Ibid.
[37] Ibid.
[38] Ibid.
[39] Ibid., 0905.
[40] Ibid., 0940.
[41] Ibid., and HESA, Evidence, 16 October 2018, 0930 (Dr. Keith Fowke, Professor, Department of Medical Microbiology and Infectious Diseases, University of Manitoba).
[42] Canadian Institutes of Health Research, Motion M-132 on using federally funded research to improve access to medicines, written submission, 22 October 2018.
[43] Drugs for Neglected Diseases initiative (DNDi), “Submission by the Drugs for Neglected Diseases initiative (DNDi), North America to the Standing Committee on Health (HESA) Study on Federally Funded Health Research (M-132),” October 2018.
[45] Ibid., 1100 (Maxwell Morgan, Director, Policy and Legal Counsel, Structural Genomics Consortium).
[49] Ibid., 0910 (Kiddell-Monroe).
[50] Ibid.
[51] Structural Genomics Consortium, “Standing Committee on Health Study M-132 Structural Genomics Consortium Briefing Note: Specific Policy Recommendations,” 17 October 2018.
[52] HESA, Evidence, 16 October 2018, 0910 (Marc LePage, President and Chief Executive Officer, Genome Canada).
[53] Structural Genomics Consortium, “Standing Committee on Health Study M-132 Structural Genomics Consortium Briefing Note: Specific Policy Recommendations,” 17 October 2018.
[54] HESA, Evidence, 18 October 2018, 0915 (Dr. Karen Lee, National Vice-President, Research, Multiple Sclerosis Society of Canada).
[58] Dr. Salim Yusuf, Distinguished University Professor of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Biomedical Research in Canada, Written submission to HESA, October 2018 and HESA, Evidence, 18 October 2018, 0855 (Nickerson).