HESA Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
CANADIANS AFFECTED BY RARE DISEASES AND DISORDERS: IMPROVING ACCESS TO TREATMENT
Introduction
On 18 April 2018, the House of Commons Standing Committee on Health (“the Committee”) adopted the following motion:
That, pursuant to Standing Order 108(2), the Committee undertake a study on the barriers to access to treatment and drugs for Canadians affected by rare diseases and disorders, including the Special Access Program, in order to develop recommendations on actions that the federal government can take, in partnership with the provinces and territories, to remove these barriers; that the Committee report its findings and recommendations to the House.[1]
As part of its study, the Committee held five meetings and heard from 24 witnesses, including government officials, physicians, researchers, pharmaceutical industry representatives and patient groups, and received 10 written submissions. Drawing on this testimony, the report begins with an overview of rare diseases in Canada and then examines the key challenges Canadians face in accessing treatment for these conditions in the following areas: regulatory approval of drugs for rare diseases; drug pricing; drug cost reimbursement through provincial and territorial drug coverage plans; and access to diagnostics. It concludes with the Committee’s observations and recommendations on how the federal government can address these challenges in collaboration with the provinces and territories.
Overview of Rare Diseases in Canada
In her appearance before the Committee, Ms. Catherine Parker (Director General, Biologics and Genetic Therapies Directorate, Health Products and Food Branch, Health Canada) explained that “rare diseases are life-threatening, debilitating or serious and chronic conditions affecting a small number of patients.”[2] Ms. Parker explained that there is no international standard definition of a rare disease. Definitions that do exist focus on determining whether the disease is rare based upon its prevalence, the proportion of a population that has the disease, or its incidence, which refers to the number of new cases of the disease that are diagnosed per year.[3] According to Ms. Parker, Health Canada has adopted a definition of a rare disease being one that affects fewer than 5 in 10,000 Canadians, which is similar to the definition adopted by the European Union. She also explained that within this definition, there are also conditions that are considered “ultra-rare,” which may affect fewer than 10 Canadians overall. In his appearance before the Committee, Dr. Joel Lexchin (professor emeritus, School of Health Policy and Management, York University) further noted that any definition of a rare disease should take into consideration not just the frequency or rarity of the disease, but also its severity or the extent to which it is debilitating.[4]
The Committee learned that approximately 80% of rare diseases are genetically based, which means that they are caused by changes or mutations in one or a few of the 20,000 genes that make up the human genome.[5] These conditions often appear at birth or in early childhood.[6] Dr. Alex MacKenzie (Clinician Scientist, Children’s Hospital of Eastern Ontario) explained that estimates suggest that there are approximately 7,000 rare diseases that have been identified to date and for approximately 94% of these diseases, no therapy or treatment is available.[7]
In terms of the impact of rare diseases on the Canadian population, witnesses provided the Committee with various figures. According to the Canadian Organization for Rare Disorders (CORD), 3 million or 1 in 12 Canadians live with a rare disease.[8] However, other witnesses including Dr. Mackenzie, Dr. Lexchin and Dr. Doug Coyle, professor, School of Epidemiology, Public Health and Preventative Medicine, University of Ottawa, suggested that this number is an over-estimation of the prevalence of rare diseases in Canada.[9] Dr. Mackenzie explained that evidence suggests that approximately one million Canadians or 2% to 3% of the population are affected by rare diseases.[10]
Dr. Mackenzie further articulated that children are disproportionately affected by rare diseases.[11] Approximately, 50% of Canadians with rare diseases are children. In addition, children with rare diseases make up one third of hospital pædiatric patients. One third of deaths in the first year of life are caused by rare diseases and one third of children with rare diseases will die before their fifth birthday. Consequently, rare diseases have a disproportionate impact in terms of years of lives lost[12] in the general population in comparison to other diseases in Canada:
Perhaps the most telling statistic is that when you look at the proportion of general years of life lost for rare diseases, it’s around 4.6%. That’s years of life lost in Canadian society. For infectious diseases, the number is around 1.4% or 1.6%. For diabetes, it’s only 2.6%. It’s really dramatic. I think the reason for this is that it takes life early on, so that carries a disproportionate impact.[13]
Finally, Dr. Mackenzie explained that approximately 50% of Canadians with a rare disease do not have a diagnosis for their condition.[14]
Barriers in Accessing Treatment for Rare Diseases in Canada: What the Committee Heard
Witnesses appearing before the Committee identified four main areas where there are barriers to accessing treatments for rare diseases: market authorization; pricing of drugs for rare diseases; reimbursement of costs for drugs for rare diseases through provincial and territorial drug coverage plans; and access to diagnostic tests for rare diseases. An overview of key challenges in these areas and possible ways that the federal government could help address these issues is provided in the sections below.
Market Authorization of Drugs for Rare Diseases
Health Canada is authorized under the Food and Drugs Act[15] and Part C, Division 8 of the Food and Drug Regulations[16] to regulate the safety, efficacy and quality of drugs, including drugs for rare diseases in Canada. Ms. Catherine Parker (Health Canada) explained to the Committee that this regulation involves overseeing the testing of new drugs in Canada through clinical trials.[17] In addition, the Department is responsible for issuing market authorizations for the sale of a new drug, once it has undertaken an assessment of both the benefits and risks of the drug to determine its quality, safety and efficacy. Finally, the Department is also responsible for monitoring the safety of these drugs once they are on the market, which is referred to as post-market surveillance. Within the context of rare diseases, Ms. Parker explained to the Committee that there are three main regulatory avenues that support Canadians’ access to drugs for rare diseases: accelerated or priority status drug approval; the Special Access Programme and clinical trials.[18]
Priority Status Drug Approval for Drugs for Rare Diseases
Ms. Parker (Health Canada) explained that the Department accelerates the approval of drugs that are intended to treat serious or life-threatening diseases, including rare diseases.[19] The Department gives them priority status or conditional approval so that patients have earlier access to them. The Committee heard that 30% to 40% of all new drugs now approved in Canada are drugs to treat rare diseases.[20] In 2017, 16 of the 36 brand name drugs approved by Health Canada are classified as orphan drugs (or drugs for rare diseases) in Europe or the United States.[21] She explained that most of these drugs were reviewed and approved using accelerated pathways. She also explained that the Department has harmonized its regulatory requirements for drug approval with those in other jurisdictions so that drug companies can file one dossier to all regulators. Health Canada and the U.S. Food and Drug Administration (FDA) now have a common portal allowing companies to file for drug approval simultaneously in both jurisdictions.[22] As a result, recent research from the Patented Medicine Prices Review Board indicates that 9 of the 10 top-selling orphan drugs in the United States are available for sale in Canada.[23] However, Ms. Parker also noted that the Department recognized the need to do more and is undertaking a review of its regulatory approval of prescription drugs, including drugs for rare diseases, to identify ways to improve its efficiency and meet patient and health care system needs.[24]
Though some witnesses[25] appearing before the Committee were generally supportive of Health Canada’s accelerated approach towards the approval of drugs for rare diseases, they also identified ways in which the Department could improve its regulatory approval process for these drugs. Dr. Craig Campbell (Pædiatric Nephrologist, Children’s Hospital, London Health Sciences Centre) articulated that regulatory agencies need to undertake a more comprehensive and flexible review of scientific evidence in their approval process for drugs for rare diseases.[26] He explained that Canadian regulatory agencies do not take into consideration all the different types of data available to evaluate the efficacy of drugs for rare diseases, such as quality-of-life data, impact on daily living, cost-analysis and meta-analysis data:
In almost every single interaction I’ve had with Canadian regulatory agencies that I’ve been a participant in, regulatory personnel have claimed that reviews for rare disease drug files can and will be done with more flexibility and be more considerate of the context and totality of the data. Further, they often claim that the existing approval processes and evidence review pathways are adaptable to rare disease drugs. However, when the final decisions are made, this rarely seems to be the case.[27]
The Committee heard that a flexible and broader approach to the review of scientific evidence for drugs for rare diseases is necessary because of the challenges associated with conducting standard clinical trials for treatments for patients with rare diseases, including the limited number of individuals who can participate in trials because of the rarity of conditions; the absence of control groups; and limited knowledge about rare diseases themselves.[28] To address this issue, Dr. Campbell recommended that Canadian regulatory bodies adopt the Canadian GRADE guidelines, which grade the quality and strength of scientific evidence and treatment recommendations.[29] He further recommended the establishment of an independent rare disease review committee that would, “help inform any regulatory agency at any level when they’re confronted with a rare disease drug review.”[30]
“[T]hey often claim that the existing approval processes and evidence review pathways are adaptable to rare disease drugs. However, when the final decisions are made, this rarely seems to be the case.”
Dr. Craig Campbell, Pædiatric Nephrologist, Children’s Hospital, London Health Sciences Centre
Given the challenges associated with the research and development (R&D) of drugs for rare diseases, other witnesses, such as representatives of the Canadian Organization for Rare Disorders (CORD), Janssen Pharmaceutical Companies, Horizon Therapeutics Canada and the Canadian Forum for Rare Disease Innovators (RAREi), recommended that Health Canada develop a specific regulatory pathway for these drugs, which has been done in other jurisdictions, such as the United States and European Union.[31] A specific regulatory framework for rare disease treatments could include incentives to develop and commercialize drugs for rare diseases in Canada, an orphan drug designation process, additional market exclusivity, research promotion funds, tax incentives and regulatory submission fee reductions.[32]
However, in his appearance before the Committee, Dr. Joel Lexchin explained that if a specific regulatory pathway for drugs for rare diseases were developed by Health Canada, it would not need to include monetary or tax incentives for the development of drugs for rare diseases, as they currently represent 37% of drugs being approved in the United States.[33] Instead, investments in research should be made for these diseases. Further, he suggested that orphan drug designations should be limited to truly unique drugs that treat only one disease rather than drugs that can be used more broadly to treat disease subsets or other diseases with similar genetic underpinnings. In addition, Health Canada should demand a high degree of rigour in clinical trials for drugs for rare diseases despite the small patient populations involved. The Department should also require post-market clinical trials for these drugs, where the evidence of their clinical benefits is not clear.[34] As noted above, Dr. Lexchin also recommended that the definition of a rare disease adopted by Health Canada include a reference to both the disease’s frequency and severity.
Special Access Programme
The Committee heard from Health Canada officials that the Special Access Programme (SAP) is another important avenue that provides patients with access to drugs for rare diseases.[35] According to Ms. Parker, Health Canada’s SAP provides access to unapproved medications on an exceptional case-by-case basis for patients with serious or life-threatening conditions when conventional treatments have failed, are unsuitable or unavailable.[36] To obtain access to drugs through this program, Dr. John Patrick Stewart (Director General, Therapeutic Products Directorate, Health Canada) explained that a physician must make a request to the program and “explain why this therapy is the best choice for the particular patient in front of them, why it’s a serious and life-threatening condition, why other therapies, if they exist have been considered and are not suitable, and evidence that supports its use.”[37] He further noted that because these requests are made only under exceptional circumstances and the drugs themselves do not go through the regular scrutiny of evidence regarding the quality, safety and efficacy of their use, they are not authorized for long periods of time, typically between three to six months. The Committee heard that approximately 30% of the drugs requested through this program are drugs for rare diseases.[38]
Witnesses appearing before the Committee saw the SAP as an important avenue for obtaining access to treatments for rare diseases.[39] However, they outlined several challenges associated with the administration of this program, which were illustrated by the problems experienced by physicians and patients who sought access to a drug to treat cystinosis in 2017. The Committee learned that cystinosis is a rare incurable, metabolic disease which effects between 75 and 100 Canadian children and young adults.[40] Without treatment, the disease results in end-stage renal failure by the time a patient reaches the average age of nine years old.[41] According to Dr. Julian Midgley, a pædiatric nephrologist, the primary treatment for cystinosis is a drug called Cystagon, which was approved by the U.S. FDA in 1994.[42] The manufacturer has never sought market authorization in Canada and consequently, patients have obtained access to this drug through the SAP for the past 20 years.
The Committee heard that in June 2017, Health Canada granted Notice of Compliance or regulatory market authorization for another drug to treat cystinosis, Procysbi.[43] Dr. Midgley explained that the new drug has the same active ingredient as Cystagon but a longer lasting formulation which improves adherence to treatment in adolescents and adults. However, he explained that there are no long-term studies of the effectiveness of the drug, which meant that many families wanted to continue to use the treatment that they knew worked for them. In addition, the cost of Procysbi is significantly higher than Cystagon at $35.05 for a 75 milligram capsule (list price), amounting to $400,000 per year in comparison to $10,000 per year for Cystagon. Once Procysbi was approved in Canada, the Committee heard from Dr. Midgely that the Department gave physicians the impression that Cystagon would no longer be available through the SAP.[44] However, once concerns were raised about the cost of Procysbi, Health Canada did continue to approve requests for Cystagon based upon medical need but did not indicate under what criteria the drug would continue to be made available through the program.[45] Furthermore, any access granted through the program has been for only three or four months, rather than the previous six months, at a time.
Ms. Erin Little, President, Liv-A-Little Foundation explained to the Committee that the uncertainty surrounding continued access to Cystagon has caused physicians, patients and their families significant duress:
When Procysbi was approved and Cystagon was so abruptly removed from Canada, and our letter of cancellation was issued, our doctor was shocked, because she had not been informed about the approval of Procysbi, and did not necessarily feel it was the best choice for her patients. When our nephrologist spoke with someone from Health Canada, giving verbal medical reasoning for Olivia to remain on her current treatment, she was denied that choice, leaving us terrified about what to do next. We were stunned that someone, however highly educated, sitting in an office, who did not know cystinosis or our child, was able to make a decision overruling our child's physician. As Olivia's primary caregiver and someone who trusts our doctors and medical system, I was disgusted that our physician was not trusted to make the most important decision for her patient.[46]
In a subsequent follow-up letter to the Committee received in January 2019, the Minister of Health provided further clarification regarding the Department’s response to providing access to Cystagon through the SAP once Procysbi became available as a marketed drug in Canada. According to the letter, Health Canada initially cancelled existing requests for Cystagon through the SAP. They then followed up with a letter to each physician who had requested Cystagon through the SAP, requesting that they provide a medical rationale as to why their patient still required the drug instead of the approved and marketed drug Procysbi. Physicians were also directly contacted by a health care professional from the SAP, who explained that they could resubmit their request through the SAP for Cycstagon instead of Procysbi, if they had a medical rationale. The letter further noted that over 60 patients have been provided access to Cystagon through the Special Access Programme since the marketing of Procysbi.
“We were stunned that someone, however highly educated, sitting in an office, who did not know cystinosis or our child, was able to make a decision overruling our child's physician. As Olivia's primary caregiver and someone who trusts our doctors and medical system, I was disgusted that our physician was not trusted to make the most important decision for her patient.”
Ms. Erin Little, President, Liv-A-Little Foundation
To address situations of these types, the Committee heard that Health Canada needs to be more proactive in their communications with physicians regarding the medical need criteria of the SAP and ensuring patients have access to drugs through this program for longer periods of time before requiring re-application.[47] In addition, patients and physicians felt that the Department needs to make a greater effort to account for patient and health care system needs in their decision-making process, such as the potential costs of medications.[48] Finally, witnesses articulated that the Cystagon case highlights the need for Health Canada to develop incentives for drug manufacturers who provide drugs through the SAP to apply for market authorization in Canada through the standard regulatory approval process.[49]
Accessing Drugs for Rare Diseases Through Clinical Trials
“Unfortunately for Norm, during this gap in treatment his disease progressed with a loss of function. It directly resulted in two significant falls. The resulting injuries required hospitalization, including epidurals, to deal with the pain from the back injury.”
Ms. Tammy Moore, Chief Executive Officer, Amyotrophic Lateral Sclerosis Society of Canada
According to Ms. Parker (Health Canada), clinical trials conducted by drug manufacturers and authorized by Health Canada also represent another opportunity for patients with rare diseases to access new treatments.[50] The Department provides drug manufacturers with advice on the design of clinical trials in small patient populations and has a clinical trial database that helps patients and primary care providers find suitable clinical trials in which to participate. While clinical trials represent an opportunity for accessing new treatments, the Committee heard that often patients face barriers in having continued access to a treatment once a clinical trial ends.[51] For example, Ms. Tammy Moore (Chief Executive Officer, Amyotrophic Lateral Sclerosis Society of Canada) told the story of Norm who had access to a drug with no adverse events through a clinical trial. While the manufacturer was willing to continue to provide the drug to him after the trial concluded, it needed to apply to Health Canada for an open-label extension to have access to the drug after the clinical trial had been completed, which can take up to six weeks. Ms. Moore explained that this time gap has negative health consequences for patients:
Unfortunately for Norm, during this gap in treatment his disease progressed with a loss of function. It directly resulted in two significant falls. The resulting injuries required hospitalization, including epidurals, to deal with the pain from the back injury.[52]
To address this issue, Ms. Moore recommended that Health Canada automatically grant an open-label extension at the conclusion of a trial, as long as there were no safety concerns during the trial, an approach that is currently taken by the FDA in the United States.
Prices Of Drugs for Rare Diseases
The Committee heard that the federal government is also responsible for regulating the prices of patented medicines, including drugs for rare diseases, through the Patented Medicine Prices Review Board (PMPRB). Mr. Douglas Clark (Executive Director, PMPRB) explained to the Committee that his organization was created in 1987 under the Patent Act through a set of reforms that aimed to encourage greater investment in pharmaceutical R&D in Canada through stronger patent protection for pharmaceuticals.[53] According to Mr. Clark:
PMPRB is a quasi-judicial body with a regulatory mandate to ensure that patentees do not abuse their patent rights by charging consumers excessive prices during the statutory monopoly period. Its creation arose out of concern that stronger patent protection for medicines might cause prices to rise unacceptably so as to become unaffordable to consumers.[54]
Mr. Clark explained that the PMPRB determines whether or not patented drug prices are excessive through an assessment that includes an evaluation of the therapeutic benefit they provide relative to existing medicines on the market. Once completed, PMPRB limits increases in the price of existing patented drugs to the rate of general inflation, and the sale price of the same drug in other seven jurisdictions, including France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States which are referred to as the “PMPRB7”.[55] The Committee heard that the PMPRB process takes approximately three months.[56] If a price is found to be excessive, the Board can then hold public hearings and order price reductions and/or the payback of excess revenues.
Despite the price ceilings set by the PMPRB, the Committee heard from witnesses that one of the main challenges in terms of accessing drugs for rare diseases is their high prices, which threaten the financial sustainability of public and private drug coverage plans.[57] In their written submission to the Committee, the pan-Canadian Pharmaceutical Alliance (pCPA), which negotiates drug prices with drug manufacturers on behalf of federal, provincial and territorial public drug plans, explained that current list prices for drugs for rare diseases range from $0.5 million to $4.9 million per person per year.[58] As these conditions are chronic in nature, treating one patient for 10 years can cost from $1 million to $49 million.[59] In its submission to the Committee, the RAREi further explained that though these drugs have a high cost per patient, their overall budgetary impact is comparatively low, representing 3.3% to 5.6% of total pharmaceutical expenditures between 2007 and 2013.[60] In addition, they are expected to remain below 6% of total pharmaceutical expenditures up to 2018. However, the Committee also heard that these drugs are expected to make up an increasing proportion of budgets of public and private drugs coverage plans in the future, as 50% of new patented medicines under the PMPRB’s jurisdiction obtained orphan drug designation in the United States or the European Union.[61] As a result, Mr. Clark explained that they pose a significant risk to the financial sustainability of the health care system:
While our system can absorb one, two or maybe even dozens of high-cost drugs, it will collapse under the weight of hundreds, no matter how good they are. At the end of the day, the single most important determinant of access is affordability. The best drug in the world won’t bring value to society if no one can afford it, or if the effect of paying for it for the fortunate few is to deprive effective health care to the multitudes.[62]
According to RAREi, manufacturers of these drugs charge high prices because of the risks and costs associated with their R&D. These risks include a lack of knowledge of rare diseases which lead to a high failure rate of treatments in clinical trials and the need to find alternative approaches to develop clinical trial data for these treatments due to small patient populations affected by the disease.[63] Most significantly, the organization explained that high prices for these drugs are necessary because they are able to recover their research and development costs from a small market due to the rarity of the condition that the drugs are treating.[64]
“The best drug in the world won’t bring value to society if no one can afford it, or if the effect of paying for it for the fortunate few is to deprive effective health care to the multitudes.”
Mr. Douglas Clark, Executive Director, Patented Medicine Prices Review Board (PMPRB)
However, the Committee heard from other witnesses, such as Drs. Lexchin, Midgley, and Coyle[65] and the pCPA, that it is also necessary for manufacturers of drugs for rare diseases to be more transparent in demonstrating the costs associated with the R&D of these drugs to justify their high prices. In its submission to the Committee, pCPA indicated that in contrast to claims made by drug manufacturers, published evidence suggests that there is considerable profit for manufacturers in the rare disease drug market. For example, a 2016 study found that orphan drugs are five times more profitable than non-orphan drugs for manufacturers.[66] Furthermore, despite the pCPA’s repeated requests for drug manufacturers to justify “their extremely high pricing, there has been no transparent justification provided to date.”[67] The organization explained that without transparent justification, payers and the public are left “with the conclusion that the prices are set based on profit maximization objectives rather than R&D recovery.”[68]
To address the high prices associated with drugs for rare diseases and other complex drugs, the Committee heard from some witnesses that is necessary to adopt a new framework for the regulation of patented drug prices through proposed amendments to the Patented Medicines Regulations that were tabled in the Canada Gazette on 2 December 2017.[69] Mr. Clark explained that there are three main types of changes being considered by the federal government through these proposed regulatory amendments.[70] First, the basket of countries used for price comparison would be expanded to include countries that have drug prices that are closer to the OECD average and health care systems and economies that are similar to Canada’s. The proposed schedule lists Australia, Belgium, France, Germany, Italy, Japan, the Netherlands, Norway, South Korea, Spain, Sweden and the United Kingdom as the new PMPRB12.[71] Second, proposed amendments would introduce new factors to determine whether a price is excessive, including the value of the drug in terms of increase in length or quality of life and potential market share of the new drug relative to Gross Domestic Product.[72] Third, manufacturers would be compelled to provide the PMPRB with information regarding confidential price rebates that they offer to public and private drug coverage payers to have a true understanding of the prices of drugs on the market.[73]
The Committee heard that drug manufacturers are opposed to these proposed regulatory changes.[74] In its submission, RAREi explained that these changes would disproportionately affect drugs for rare diseases, resulting in price reductions of between 70‑90%. Furthermore, the organization explained that the proposed approach for determining the value of the drug or cost-effectiveness of a drug does not take into account limitations associated with developing scientific evidence for drugs for rare diseases, as noted above. RAREi indicated that these changes would create barriers for manufacturers seeking market approval in Canada, causing delays and/or limiting access to treatments for patients altogether by no longer launching products in the Canadian market. It therefore recommended that the federal government reconsider this regulatory proposal.[75]
Mr. Clark explained to the Committee that the PMPRB is aware of concerns that the proposed changes to the PMPRB regulatory framework might delay or compromise Canadians’ access to the very latest patented drugs.[76] However, he cautioned the Committee that there is little evidence to support the argument that lower prices result in less access. According to Mr. Clark, “the reality is that many countries with similar health care systems and economies to Canada’s pay less for drugs yet enjoy the same or better access. The same is true of research and development investment.”[77]
The Committee heard that Canada currently pays the third-highest price for patented medicines in the world, and yet the ratio of research and development to sales in Canada pales in comparison to the PMPRB7 countries. [78] According to Mr. Clark:
[T]he original composition of that group of countries was based upon the assumption that if we emulated the intellectual property regimes in those countries and priced in line with them, we would come to enjoy a similar level of R and D to sales as in those countries. Obviously, that assumption has not been borne out over time. Currently, we are at an historical low in our ratio of R and D to sales.… It currently stands at about 4.4%, versus over 20% R and D to sales in the countries we compare ourselves to on average under the PMPRB7.[79]
According to Mr. Clark, the PMPRB is “not doing a very good job” of protecting Canadians from excessive pricing with the tools currently at its disposal and it is for this reason that the PMPRB is seeking to move forward with ambitious reforms to its pricing guidelines.[80] First announced in June of 2016, these changes were set to take effect on 1 January 2019 but they have now been postponed indefinitely.[81]
Reimbursement of Rare Disease Drug Costs Through Federal Provincial and Territorial Public Drug Coverage Plans
“Obviously, that assumption has not been borne out over time. Currently, we are at an historical low in our ratio of R and D to sales.… It currently stands at about 4.4%, versus over 20% R and D to sales in the countries we compare ourselves to on average under the PMPRB7.”
Mr. Douglas Clark, Executive Director, Patented Medicine Prices Review Board (PMPRB)
The Committee heard that provinces and territories are responsible for determining which drugs will be reimbursed through their respective public drug coverage plans, including drugs for rare diseases.[82] In addition, the federal government offers or facilitates public drug coverage for some groups of people, including members of the military, veterans, First Nations and Inuit, federal inmates, and certain classes of refugees.[83] In its submission to the Committee on behalf of public drug plans in Canada, the pCPA explained that each plan aims to deliver coverage that is accessible, appropriate, affordable and responsive to population health needs.[84] These plans are also responsible for funding other aspects of the health care system, including medical devices and supplies as well as pharmacy services. Finally, they are also publicly accountable for the use of tax payer funds and must make trade-offs in terms of which drugs and services will be funded within health care systems that have a significant number of competing health priorities but limited budgets.
In its submission, pCPA explained that federal, provincial and territorial public drug coverage plans rely on national and pan-Canadian processes to support their decision-making, including the Canadian Agency for Drugs and Technologies in Health (CADTH) and the pan-Canadian Pharmaceutical Alliance (pCPA) (see Figure 1).[85] The aim of both CADTH and the pCPA is to establish consistency in pricing and reimbursement decisions for drugs across the country.[86] CADTH is an independent not-for-profit corporation that was established by federal, provincial and territorial governments (except Quebec)[87] in 1989 to provide them with advice and guidance to determine which drugs and medical technologies should be reimbursed through public health care plans.[88] Ms. Heather Logan (Acting Vice-President, Pharmaceutical Reviews, CADTH) explained to the Committee that once a drug has been authorized for sale by Health Canada, CADTH then provides advice and recommendations to public drug coverage plans by undertaking a health technology assessment (HTA), which is an evidence-based review of both the clinical- and cost-effectiveness of drugs.[89] Evaluating the cost-effectiveness of a drug involves looking at the clinical benefit of a drug in terms of health outcomes, such as quality of life, morbidity, and mortality, in comparison to its cost to determine whether it represents value for money. HTAs also examine the cost-effectiveness of a drug in comparison to other treatments available. They also identify specific patient and clinical circumstances when the drug works best.[90] Through the Common Drug Review, CADTH provides recommendations for drug coverage decisions for 18 of the 19 publicly funded drug coverage plans in Canada, including federal programs. CADTH has a similar but separate process for making recommendations for oncology drugs through the pan-Canadian oncology drug review.[91]
Figure 1
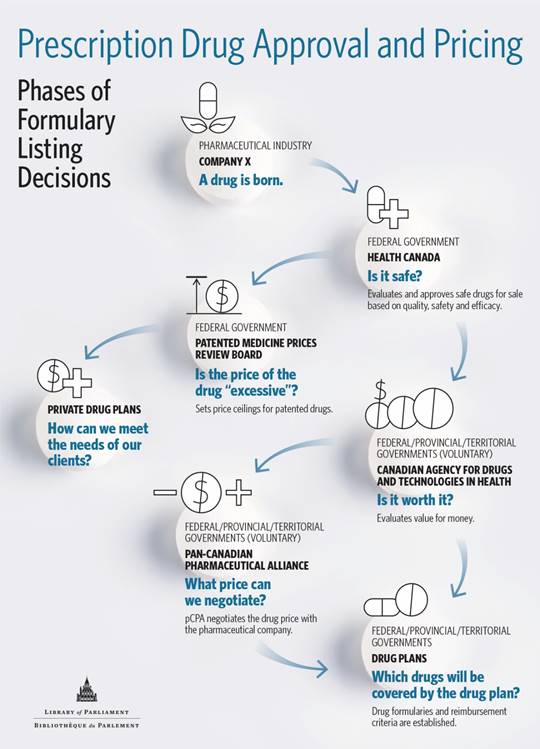
Once a drug has undergone CADTH’s assessment process and received a positive recommendation, the pCPA, on behalf of provincial, territorial and federal drug coverage plans, negotiates with drug manufacturers to obtain price reductions and other conditions for the listing of a drug on public drug coverage plans.[92] Once these negotiations are complete, jurisdictions then enter into their own respective product listing agreements with drug manufacturers. As of 30 September 2018, the pCPA had completed 200 negotiations for both brand-name and generic medicines, realizing a savings of $1.98 billion.[93] As of 26 November 2018, the organization had completed nine negotiations for drugs for rare diseases.[94]
The Committee heard that both CADTH and the pCPA face difficulties in making drug coverage recommendations and undertaking price negotiations for drugs for rare diseases because of the limited clinical data for these drugs. As the target population for these drugs is small, it is difficult to conduct a standard clinical trial.[95] When less than robust clinical trial data for these drugs exist, there is a high degree of uncertainty around the clinical benefit and long-term safety, efficacy and cost-effectiveness of the treatment. Further, the clinical data for these drugs use surrogate end-points such as changes to biomarkers or biological processes, which may correlate with improvements in health outcomes, such as quality of life or reduction in mortality, but are not proven to result in better health outcomes.[96]
Finally, pCPA, in its brief, also explained that when Health Canada approves a drug for sale with conditions, such as the need to collect more evidence to support its efficacy, there are limited consequences for pharmaceutical companies who do not fulfil these obligations.[97] Consequently, when these drugs are later assessed by CADTH and the pCPA, they are unable to evaluate the efficacy of a drug because this data is missing or limited. The unclear evidence regarding the clinical benefits or harms of drugs for rare diseases coupled with their high costs therefore makes it difficult for public drug plans to justify their reimbursement. The pCPA explained that there are some drugs for rare diseases that have adequately demonstrated their clinical efficacy sufficiently to support their reimbursement with conditions, which usually incude a price reduction and specific clinical criteria for use of the drug.[98]
To address challenges associated with scientific evidence regarding the clinical efficacy and cost-effectiveness of drugs for rare diseases, Ms. Logan (CADTH) recommended investing in infrastructure and support for the collection of real world evidence.[99] This support would enable public drug plans to provide conditional coverage of a drug when there is limited evidence; and then reassess or renegotiate with drug manufacturers when additional evidence is available, a managed access approach to rare disease drugs that is used in other jurisdictions.[100] Dr. Campbell, Children’s Hospital, London Health Sciences Centre echoed this view, recommending investment in rare disease registries in which expert clinicians collect long-term, high-quality patient data on health outcomes and biomarkers to monitor the impact of novel therapies.[101]
The Committee heard that there is also a need to improve the timeliness of drug reimbursement processes through CADTH and the pCPA through better coordination among these bodies and Health Canada’s market approval process to improve access to drugs for rare diseases. Mr. Andrew McFadyen (Executive Director, the Isaac Foundation) explained that despite Health Canada’s priority review process, patients are still often left waiting for access to a drug because they must also wait for both CADTH’s review, which could take an additional 6 to 12 months and pCPA negotiations, which can take 12 months or longer.[102] Ms. Tammy Moore (Chief Executive Officer, Amyotrophic Lateral Sclerosis Society of Canada) explained that this lengthy timeline for regulatory approval and drug cost reimbursement processes through CADTH and the pCPA in Canada is particularly detrimental to patients with rare diseases because their conditions can decline rapidly without access to treatment:
In the 180 days during Health Canada’s priority review period, 500 Canadians have died of ALS. How many will die awaiting the CADTH decision? After that, how many will have to die while they’re awaiting the availability through a publicly funded drug program? We are dealing with a community that measures time by loss of their own function and the number of members who will die during this process.[103]
To reduce the timeliness associated with drug approval and reimbursement processes, Mr. McFadyen recommended a more coordinated drug approval and reimbursement process in which Health Canada and CADTH reviews would take place simultaneously, and pCPA negotiations would also begin at the same time.[104] The Committee heard from Ms. Parker that Health Canada is starting to move in this direction, working with CADTH to have their respective review processes occur in tandem.[105] In its brief, pCPA recommended that Health Canada, in collaboration with CADTH and the pCPA, provide advice and support to drug manufacturers regarding the design of clinical trials to ensure that they are able to meet the scientific evidence requirements for both drug approval by Health Canada and the price negotiation and cost reimbursement processes of provincial and territorial drug plans.[106]
“In the 180 days during Health Canada’s priority review period, 500 Canadians have died of ALS. How many will die awaiting the CADTH decision? After that, how many will have to die while they’re awaiting the availability through a publicly funded drug program? We are dealing with a community that measures time by loss of their own function and the number of members who will die during this process.”
Ms. Tammy Moore, Chief Executive Officer, Amyotrophic Lateral Sclerosis Society of Canada
The Committee also heard from witnesses that it is necessary to provide time-limited coverage for the costs of drugs for rare diseases for patients during the approval and reimbursement processes to ensure that they have uninterrupted access to these drugs.[107] The Committee heard that some drug manufacturers cover the costs of medications for patients while the drugs are in the regulatory approval processes through compassionate care programs. However, these programs also pose challenges for patients and public drug coverage plans. Ms. Little, President, Liv-A-Little Foundation explained that once patients are part of these programs, they may not be able to obtain coverage for the drug from their private insurance companies or are unable to switch drugs, if a new one comes on the market.[108] A representative from the pCPA indicated that it had experienced numerous situations where manufacturers threatened to stop their compassionate supply as part of their negotiating position with public drug plans.[109] Mr. McFadyen therefore recommended that the federal government set aside a very small percentage of health care transfers to the provinces and territories to provide immediate access to lifesaving drugs that have been approved by Health Canada, but are still undergoing the pricing negotiation and cost-reimbursement process through CADTH and the pCPA.[110]
More broadly, some witnesses told the Committee that patients should be provided with equitable access to drugs for rare diseases through either a national drug coverage program for drugs for rare diseases, or a national pharmacare program for all drugs that would include drugs for rare diseases. In the words of Ms. Maureen Smith (Board Secretary, CORD):
I have a lot of ideas about recommendations, but I think the thing that strikes me the most is that there is no equity across the country. It’s very difficult for a patient to know that someone in B.C. or someone in another province has exactly the same condition as you and receives treatment, when maybe in your province, you don’t. People who don’t have rare diseases or who don’t deal with drugs are flabbergasted by that. We’re all Canadians, and they seem to think that universal coverage in hospitals extends to drugs. There’s very little understanding of that until you are in that situation yourself.[111]
“It’s very difficult for a patient to know that someone in B.C. or someone in another province has the exactly the same condition as you and receives treatment, when maybe in your province, you don’t. People who don’t have rare diseases or who don’t deal with drugs are flabbergasted by that. We’re all Canadians.…”
Ms. Maureen Smith, Board Secretary, CORD
Finally, the Committee heard that some progress is currently underway to address these issues. Ms. Logan explained that the Expensive Drugs for Rare Disease (EDRD) working group was established by provincial and territorial deputy ministers of health in 2014 to explore ways of managing access to these drugs.[112] The working group has developed a proposal that would involve creating a supplemental process for drugs for rare diseases that would involve:
- a co-ordinated identification and prioritization mechanism for the regulatory review of complex drugs;
- improved use of real-world evidence to inform regulatory and reimbursement decisions;
- centralized panels of experts to evaluate evidence and ensure consistency in decision-making; and
- time-limited access as additional clinical evidence is being gathered.[113]
The Committee heard that consultations regarding this proposal were underway in November 2018. In their written submissions to the Committee, the pCPA and drug manufacturers recommended that the federal government support the supplementary process proposed by the EDRD working group.[114]
Access to Early Diagnosis of Rare Diseases
Dr. Michael Brudno, (professor and Scientific Director, Centre for Computational Medicine, Hospital for Sick Children) told the Committee that obtaining early diagnosis of a rare diseases is critical for patients to access treatment.[115] He explained that genetic testing is available to identify disease-causing mutations and differentiate among 7,000 diseases at a cost of $1,000 per test.[116] He explained that rapid access to genetic testing means that patients gain access to treatment sooner, avoiding unnecessary visits to doctors, investigations and interventions that can cost the health care system upwards of US$8,000 per patient based upon studies conducted in the United States. Dr. Brudno recommended that any approach to address barriers to access to treatment for rare diseases in Canada should include access to rapid diagnostic genetic testing as a critical component. Dr. Alex MacKenzie (Clinician Scientist, Children’s Hospital of Eastern Ontario) also recommended that the federal government continue to invest in research initiatives such as Genome Canada’s 30,000 genome identification project and the pan-Canadian research consortium Care4Rare that is supporting the identification of rare disease genes.[117]
The Way Forward: Committee Observations And Recommendations
The Committee’s study highlighted the numerous challenges that Canadians with rare diseases face in accessing treatments. The Committee heard that current approval, pricing and reimbursement processes for drugs for rare diseases are not meeting the needs of patients, drug manufacturers and provincial and territorial public drug coverage plans. The result is significant tension among different parties within the health care system:
As it stands, many families have to parade their children through the media. Many drug companies are manipulating physicians and the public. Clinicians and patient groups are reacting in shock to decisions. Regulatory bodies appear to be stifled in real engagement by their internal bylaws and processes.[118]
It is clear to the Committee that something needs to change. With more and more drugs for rare diseases coming to the Canadian market, we need to address their high prices and limitations in the scientific evidence supporting their use, while ensuring that patients have continued access to treatment. In addition, manufacturers must be able to obtain a fair return on their investments and continue to have incentives to seek market authorization in Canada. Witnesses appearing before the Committee highlighted ways in which the federal government, in collaboration with provinces and territories, could help balance these competing priorities and improve access to treatments for Canadians with rare diseases. The witnesses identified the need for a distinct and coordinated process for both the market authorization and reimbursement of these drugs across all levels of government. Such a process would ensure timely access to drugs for rare diseases, which would include a broad and more flexible consideration of scientific evidence in relation to these drugs. Witnesses also indicated that Health Canada needs to do a better job of communicating with physicians and patients regarding their regulatory criteria and decisions, particularly as they relate to the Special Access Programme. Health Canada also needs to provide guidance to drug manufacturers to ensure that they can provide the scientific evidence required for both market approval and reimbursement of drugs for rare diseases.
Finally, witnesses articulated that the single most important factor for ensuring access to drugs for rare diseases is affordability. Consequently, the Committee heard that it is necessary to implement the proposed amendments to the Patented Medicines Regulations to reduce drugs prices in Canada and ensure the financial sustainability of public health care systems. Witnesses also recommended federal funding for short and long-term public drug coverage programs for drugs for rare diseases to ensure access for patients. Meanwhile public investments in research on rare diseases will help in the development of new treatments and help monitor the effectiveness of existing ones.
The Committee agrees with the proposals put forward by witnesses during its study and therefore recommends:
Health Canada’s Market Authorization of Drugs for Rare Diseases
Recommendation 1
That the Government of Canada, in collaboration with the provinces and territories, develop a coordinated process for the market authorization and reimbursement of drugs for rare diseases.
Recommendation 2
That the Government of Canada work to ensure greater transparency and information sharing throughout the life cycle of drugs for rare diseases to ensure timely access for key decision-makers, including health care providers, health technology assessors and patients.
Recommendation 3
That the Government of Canada in collaboration with the provinces and territories develop a national, independent, expert review panel to provide recommendations and guidance on the regulatory review, pricing and reimbursement of drugs for rare diseases in Canada, including instructions on how to streamline these processes; and report publicly on its findings.
Recommendation 4
That Health Canada and the Canadian Agency for Drugs and Technologies in Health undertake their respective scientific evidence review processes of drugs for rare diseases in tandem as a standard practice.
Recommendation 5
That Health Canada, in collaboration with the Canadian Agency for Drugs and Technologies in Health, provide guidance and advice to drug manufacturers in the design of clinical trials to ensure that they meet the requirements of both market authorization and reimbursement processes in Canada.
Recommendation 6
That Health Canada consider removing regulatory requirements for drug manufacturers to seek additional approval for an open-label extension for drugs at the completion of a clinical trial to ensure that patients have uninterrupted access to these drugs if no safety concerns are present, in line with regulatory practices in the United States.
Recommendation 7
That Health Canada consider reducing regulatory submission fees for manufacturers of drugs for rare diseases seeking to obtain market authorization for the drugs in Canada.
Recommendation 8
That Health Canada be more proactive in its communications with physicians and patients regarding the specific medical need criteria required for obtaining access to drugs through the Special Access Programme.
Recommendation 9
That the Government of Canada remove the requirement to reapply to the Special Access Programme every three to six months when accessing a drug for a permanent, stable condition. Once initially approved, Canadians' approvals should remain in place until a doctor rescinds the approval or the patient's condition changes significantly.
Recommendation 10
That Health Canada ensure that drug manufacturers meet their regulatory obligations when Notice of Compliance with conditions are granted for drugs where limited evidence is available regarding their quality, safety and efficacy.
Drug Prices
Recommendation 11
That the Government of Canada move forward with implementing proposed changes to the Patented Medicines Regulations to address high drug prices in Canada.
Recommendation 12
That the Government of Canada consider establishing separate requirements for determining price ceilings for drugs for rare diseases under the Patented Medicines Regulations to reflect the small market for these drugs in Canada.
Recommendation 13
That the Patented Medicine Prices Review Board be required to consider the advice and recommendations of the proposed independent advisory committee on drugs for rare diseases in setting the price ceilings for drugs for rare diseases.
Recommendation 14
That the Government of Canada introduce additional regulatory requirements under section 88(1) (c) of the Patent Act that require manufacturers of patented pharmaceuticals to provide information to the Patented Medicine Prices Review Board regarding their research and development costs for a drug once they have obtained market authorization from Health Canada.
Recommendation 15
That the Government of Canada undertake a review of the entire pharmaceutical research and manufacturing process to better understand where government regulations and laws are having the unintended consequences of raising final drug costs for patients. This review should include an examination of whether drug costs could be reduced through open science.
Reimbursement of Drugs for Rare Diseases
Recommendation 16
That the Government of Canada, in collaboration with the provinces, territories and drug manufacturers, establish a jointly funded compassionate care program that covers the costs of drugs for rare diseases while they are under review for market authorization and cost reimbursement.
Recommendation 17
That the reimbursement of drugs for rare diseases be included as part of a national pharmacare program established by the Government of Canada, in collaboration with the provinces and territories, through amendments to the Canada Health Act, as recommended by the House of Commons Standing Committee on Health in its report entitled Pharmacare Now: Prescription Medicine Coverage For All Canadians.
Recommendation 18
That the Office of the Auditor General conduct an audit of Health Canada to determine whether it has been effective in managing its funding agreement with the Canadian Agency for Drugs and Technologies in Health, including determining whether Health Canada is effectively ensuring that the Agency is fulfilling its mandate in accordance with agreed terms and conditions of the agreement with Health Canada.
Research
Recommendation 19
That the Government of Canada provide funding through the Canadian Institutes of Health Research for research into the diagnosis of patients with rare diseases and the collection of real-world evidence regarding the effectiveness of treatments for these conditions.
[1] House of Commons Standing Committee on Health (HESA), Minutes of Proceedings, 1st Session, 42nd Parliament, 18 April 2018.
[2] HESA, Evidence, 27 September 2018, 0900 (Ms. Catherine Parker, Director General, Biologics and Genetic Therapies Directorate, Health Products and Food Branch, Department of Health).
[3] Ibid.
[4] HESA, Evidence, 25 October 2018, 0835 (Dr. Joel Lexchin, professor emeritus, School of Health Policy and Management, York University, as an Individual).
[5] Ibid., 0830 (Dr. Michael Brudno, professor and Scientific Director for Computational Medicine, Hospital for Sick Children).
[7] HESA, Evidence, 25 October 2018, 0835 (Dr. Alex MacKenzie, Clinician Scientist, Children’s Hospital of Eastern Ontario (CHEO)).
[8] HESA, Evidence, 27 September 2018, 0945 (Dr. Durhane Wong-Rieger, President and Chief Executive Officer, Canadian Organization for Rare Disorders).
[9] HESA, Evidence, 25 October 2018, 0835 (Lexchin, Mackenzie) and HESA, Evidence, 4 October 2018, 1005 (Dr. Doug Coyle, professor, School of Epidemiology, Public Health and Preventative Medicine, University of Ottawa, as an Individual).
[10] Ibid., 0835 (MacKenzie).
[11] Ibid.
[12] Years of lives lost (YLL) measures the number of years of life lost to a cause as a proportion of the total YLL lost in the population due to premature mortality. YLL is calculated by multiplying the number of deaths by a standard life expectancy at the age at which death occurs. By taking into account the age at which death occurs, this indicator give greater weight to diseases that result in loss of life at an early age. WHO, Years of lives lost (percentage of total).
[13] Ibid.
[14] Ibid.
[15] Food and Drugs Act, R.S.C., 1985, c. F-27.
[16] Food and Drug Regulations, C.R.C., c. 870.
[18] Ibid.
[19] Ibid.
[20] Ibid.
[21] Ibid.
[22] Ibid., 0940.
[23] Ibid., 0905.
[24] Ibid.
[25] Please see for example, HESA, Evidence, 27 September 2018, 0945 (Wong-Rieger) and HESA, Evidence, 4 October 2018, 0905 (Mr. Andrew McFadyen, Executive Director, the Isaac Foundation).
[26] HESA, Evidence, 4 October 2018, 0915 (Dr. Craig Campbell, Pædiatric Nephrologist, Children’s Hospital, London Health Sciences Centre).
[27] Ibid.
[28] HESA, Evidence, 27 September 2018, 0915 (Dr. John Patrick Stewart, Director General, Therapeutic Products Directorate, Department of Health) and The Canadian Forum for Rare Disease Innovators (RAREi), “Unique approach needed: Addressing barriers to accessing rare disease treatments,” written submission to HESA, 20 November 2018.
[30] Ibid., 0920.
[31] HESA, Evidence, 27 September 2018, 0945 (Wong-Rieger); (RAREi), “Unique approach needed: Addressing barriers to accessing rare disease treatments,” written submission to HESA, 20 November 2018; Horizon Therapeutics Canada, “Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission, October 2018; Janssen Pharmaceutical Companies, “Janssen submission to the House of Commons Standing Committee on Health’s consultations on barriers to access to treatment and drugs for Canadians affected by rare diseases and disorders,” written submission, 25 October 2018.
[32] RAREi, “Unique approach needed: Addressing barriers to accessing rare disease treatments,” written submission to HESA, 20 November 2018.
[34] Ibid.
[36] Ibid.
[39] Ibid., 0945 (Wong-Rieger); RAREi, “Unique approach needed: Addressing barriers to accessing rare disease treatments,” written submission to HESA, 20 November 2018; and Horizon Therapeutics Canada, “Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission, October 2018.
[40] HESA, Evidence, 4 October 2018, 0845 (Dr. Julian Midgley, Pediatric Nephrologist, As an Individual).
[43] Ibid.
[44] Ibid.
[45] Ibid.
[47] HESA, Evidence, 4 October 2018, 0850 (Midgley) and RAREi, “Unique approach needed: Addressing barriers to accessing rare disease treatments,” written submission to HESA, 20 November 2018.
[48] HESA, Evidence, 4 October 2018, 0850 (Midgley) and HESA, Evidence, 30 October 2018, 0910 (Little).
[50] Ibid., 0900 (Parker).
[51] Ibid., 1005 (Ms. Tammy Moore, Chief Executive Officer, Amyotrophic Lateral Sclerosis Society of Canada).
[52] Ibid.
[53] HESA, Evidence, 6 November 2018, 0850 (Mr. Douglas Clark, Executive Director, Patented Medicine Prices Review Board (PMPRB)).
[54] Ibid.
[55] Ibid and PMPRB, Annual Report, 2017.
[57] Ibid and pan-Canadian Pharmaceutical Alliance (pCPA), “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[58] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018, p. 4.
[59] Ibid.
[60] RAREi, “Unique approach needed: Addressing barriers to accessing rare disease treatments,” written submission to HESA, 20 November 2018.
[62] Ibid., 0855 (Clark).
[63] RAREi, “Unique approach needed: Addressing barriers to accessing rare disease treatments,” written submission to HESA, 20 November 2018, pp. 3-6.
[64] Ibid., p. 6.
[65] HESA, Evidence, 4 October 2018 (Midgley, Coyle); HESA, Evidence, 25 October 2018, 0840 (Lexchin); and pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[66] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[67] Ibid., p. 5.
[68] Ibid.
[69] Ibid and HESA, Evidence, 4 October 2018, 0900 (Coyle) and HESA, Evidence, 6 November 2018, 0850 (Clark).
[71] Canada Gazette, “Regulations Amending the Patented Medicines Regulations,” Vol. 151, No.48-December 2, 2017.
[73] Ibid.
[74] RAREi, “Unique approach needed: Addressing barriers to accessing rare disease treatments,” written submission to HESA, 20 November 2018, p. 7.
[75] Ibid.
[77] Ibid.
[78] Ibid.
[79] Ibid., 0905.
[80] Ibid., 0925.
[81] The Globe and Mail, “Canada’s drug-pricing regulator brings rare allegation of excessive pricing against Horizon Pharma,” 18 January 2019.
[82] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[83] Ibid.
[84] Ibid.
[85] Ibid.
[86] Ibid.
[87] Quebec has its own separate HTA process conducted by the Institut national d’excellence en santé et en services sociaux.
[88] HESA, Evidence, 6 November 2018, 0855 (Ms. Heather Logan, Acting Vice-President, Pharmaceutical Reviews, Canadian Agency for Drugs and Technologies in Health).
[89] Ibid.
[90] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[92] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[93] Ibid.
[94] Ibid.
[96] Ibid.
[97] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[98] Ibid.
[99] HESA, Evidence, 6 November 2018, 0950 (Logan) and pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[100] Ibid.
[102] Ibid., 0910 (McFadyen).
[106] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[109] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[113] pCPA, “Brief to House of Commons Standing Committee on Health: Barriers to Access Treatment and Drugs for Canadians Affected by Rare Diseases and Disorders,” written submission to HESA, 7 December 2018.
[114] Ibid.
[116] Ibid.
[117] Ibid., 0835 (MacKenzie).